About the origin of the acrocentric part of non-acrocentric satellited chromosomes in humans
Aннотация
Background: Variants in size of the acrocentric short arms (acro-ps) are normally not reported and considered as chromosomal heteromorphisms (CHMs) without any influence on the carrier’s phenotype. However, if acro-ps are translocated to ends of A-chromosomes (i.e. human chromosomes 1-22 and X or Y), those rearrangements are studied in more detail. The aim of the study: Here we characterized 11 healthy carriers of a non-acrocentric satellited chromosomes der(A)t(A;acro)(pter or qter;p1?1.2) to determine the frequency of chromosome 15p and 22p in such rearrangements. Materials and methods: 11 carriers of one (10 cases) or two (1 case) der(A)t(A;acro) were identified during routine cytogenetic analyses. They were originally referred due to infertility or due to a mentally retarded child with otherwise abnormal karyotype. Here derivative chromosomes were studied by fluorescence in situ hybridization applying probes D15Z1 (specific for 15p11.2) and D22Z4 (specific for 22p11.2). As there are no DNA-sequences available for 13p11.2, 14p11.2 and 21p11.2 these regions could not be tested. Results: D15Z1 sequences were identified in 1 out of 12 derivatives der(A)t(A;acro). D22Z1 could not be detected in any of the 11 remainder derivatives. However, only 3 of the 12 der(A)t(A;acro) had acro-ps large enough to potentially comprise sub-band p11.2. Conclusion: In contrast to der(Y)t(Y;acro)(q12;p1?1.2), where in at least 65% of the cases the acro-p part contains D15Z1 sequences, here it could be shown that in der(A)t(A;acro) 15p involvement can be substantiated much less frequently. Also, in none of the two groups D22Z4-sequences were detected in acro-p-parts yet. Besides, breakpoint of acro-p-parts in der(A)t(A;acro) seem to be in ~75% of the cases distal from p11.2.
Ключевые слова: acrocentric short arms (acro-ps), chromosomal heteromorphisms (CHMs), D15Z1 (specific for 15p11.2), D22Z4 (specific for 22p11.2)
К сожалению, текст статьи доступен только на Английском
Introduction. Chromosomal heteromorphisms (CHMs) are considered as cytogenetically detectable gross chromosomal aberrations from the norm, which nonetheless do not lead to any instantaneous clinical consequences. Such changes include euchromatic variants (EV) [1] as well as variations in size and location of heterochromatic DNA-stretches [2]. They can be passed through generations and rarely their new formation has been documented [3].
Typical CHMs, being visible in practically each human karyogram, are size-variations of the acrocentric short arms (acro-ps), i.e. in the overall ten p-arms of chromosomes 13, 14, 15, 21 and 22. In the “International System for Human Cytogenomic Nomenclature” (ISCN, 2020) it is recommended not mentioning them in genetic reports [4]. Acro-ps typically carry a nucleolus organizing region (NOR) in sub-band p12 with ~40 copies, making up a total of 300 - 400 copies per cell [5].
A specific form of CHM involving acro-ps is the presence of an additional, eleventh acro-p being attached at the very tip of another A-chromosome (= in human the chromosomes 1-22 and X or Y). In case if a normal carrier, (almost) no euchromatic material is lost at the telomeric end of the affected A-chromosome, which can be described as der(A)t(A;acro)(pter or qter;p1?1.2) or non-acrocentric satellited chromosomes. Such derivative chromosomes are only found unexpectedly in infertile diagnostics or parental studies in an otherwise affected child [6]. The most frequently observed der(A)t(A;acro) is the der(Y)t(Y;acro)(q12;p1?1.2); therefor a recent study revealed that at least 65% those derivatives are indeed a der(Y)t(Y;15)(q12;p11.2) [7].
Accordingly, here we studied 11 healthy carriers of one (10 cases) or two (1 case) der(A)t(A;acro)(pter or qter;p1?1.2) to determine the frequency of chromosome 15p and 22p in such rearrangements. Chromosomes 15 and 22 were chosen, as probes are available for 15p11.2 and 22p11.2, and not for 13p11.2, 14p11.2 or 21p11.2.
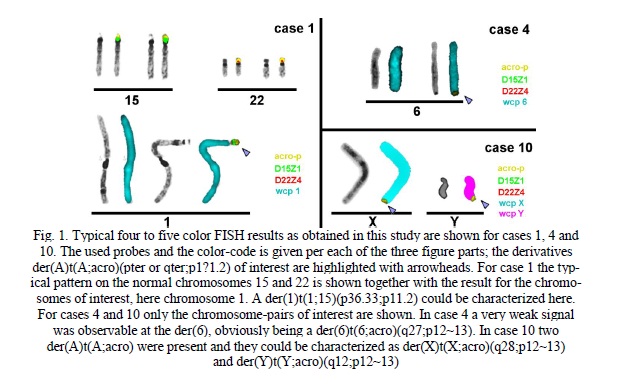
Materials and Methods. Chromosomal preparations were derived from PHA-stimulated, cultivated lymphocytes of seven individuals with different indications as listed in Table 1. Karyotyping (= GTG-banding) and FISH were done according to standard procedures [8]. For the latter the following probes were applied: 15p11.2 (D15Z1) (Abbott/Vysis, Wiesbaden, Germany), 22p11.2 (D22Z4) [9], a probe specific for all acrocentric short arms (acro-p = midi54 – microdissection derived probe [10]), whole chromosome painting (wcp) probes 1, 4, 6, 13, 18, 20, 22, X and Y [10]. Four- to five-color-FISH was done as shown in Fig. 1. Probe D15Z1 was labeled in SpectrumGreen, acro-p in Cyanine 5, D22Z4 in SpectrumOrange, wcp probes in diethylaminocoumarine and in case 10 the wcp probe for Y-chromosome in TexasRed.

Ethics Statement
The patients were recruited and studied in frame of routine clinical genetics diagnostics.
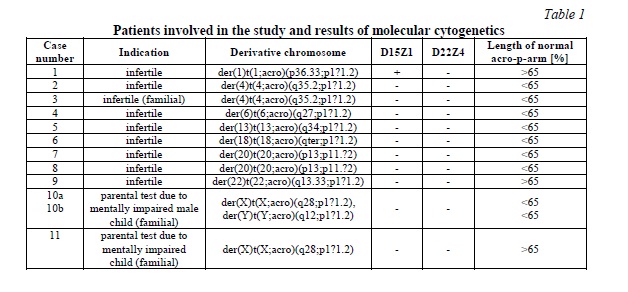
Results. In 11 cases with overall 12 derivatives only in case 1 der(A;acro) comprised material derived from a short arm of chromosome 15. Thus, there a der(1)t(1;15)(p36.33;p11.1) could be characterized. In none of the remained 11 der(A;acro) D15Z1 or D22Z4 material could be detected.
Thus, the cases were further analyzed for the length of the acro-p part on the der(A;acro) compared to the other acro-ps within the same case. According to the evaluation scheme, that sub-band p11.2 can only be comprised in der acro-p arm if it is larger than 65%, there remained only 3/12 der(A;acro) being large enough to expect a FISH-result (cases 1, 9 and 11). Accordingly, in all those cases the karyotypes have to been revised to der(A)t(A;acro)(pter or qter;p12~13).
Discussion. CHMs are clearly understudied. Just recently we could show that at least 65% of der(Y)t(Y;acro)(q12;p1?1.2) can indeed be described as der(Y)t(Y;15)(q12;p11.2) [7]. Corresponding data for other rare cases with der(A)t(A;acro) were not available yet.
Here it could be shown that most of der(A)t(A;acro) have the acrocentric breakpoint rather in p12~13 than in p11.1~11.2. This hampers also for future studies their clear attribution to a chromosomal origin, as also e.g. tried without much success be other by a- or ß-satellite probes [6]. As in 3 out of 12 der(A)t(A;acro) the acrocentric breakpoint seemed to be in p11.2 or even p11.1, it may be deduced carefully that other than for der(Y)t(Y;acro) cases D15Z1 is less often involved in der(A)t(A;acro).
It is well known that non-deleterious mutations / aberrations in the Y-chromosome can be by far easier spread in a population than such on other human chromosomes. Thus, it is logical to find der(Y)t(Y;acro) more often than all other variants of der(A)t(A;acro). Also, for the latter variant even a founder effect is described for Canadian population [11].
For der(A)t(A;acro) no founder effects were reported yet, still in the present study 3 of 11 cases (cases 3, 10 and 11) were familial (Tab.1), as were also other described in literature [2, 6].
Conclusion. In conclusion, together with our recent previous work [7] the enigma of origin of the acrocentric part of non-acrocentric satellited chromosomes in humans could be a bit more enlighted. While in der(Y)t(Y;acro)(q12;p1?1.2) in at least 65% of the cases the acro-p part contains D15Z1 sequences, here it could be shown that in der(A)t(A;acro) the 15p involvement can be substantiated much less frequently. Also, in none of the two groups D22Z4-sequences were detected in acro-p-parts yet. Besides, breakpoint of acro-p-parts in der(A)t(A;acro) seem to be in ~75% of the cases distal from p11.2.
Благодарности
Clinical cases were provided by Dr. Gödde (Recklinghausen, Germany), Dr. Hehr (Regensburg, Germany), Dr. Hentze (Heidelberg, Germany), Dr. Kläs (Mannheim, Germany), Dr. Lemmens (Aachen, Germany), Drs. Wagner and Stibbe (Hannover, Germany), Dr. Manolakis (Athens, Greece). The probe D22Z4 was kindly provided by Prof. Mariano Rocchi, Bari, Italy




















Список литературы