Correction of hypertensive retinal changes in rats with Semax
Aннотация
Background: Currently, there are no drugs for the specific treatment of hypertensive retinal changes. The main therapy is for the treatment of a systemic disease – hypertensive disease. Therefore, the search for ways of specific pharmacological correction of hypertensive retinal changes is of great interest. The aim of the study: To evaluate the correction possibility of retinal injuries with Semax in a rat model of hypertensive neuroretinopathy. Materials and methods: The model was performed by injection of N-nitro-L-arginine methyl ester (L-NAME) at a dose of 1.25 mg/100 g of rat mass within 28 days and a single increase in intraocular pressure (IOP) to 110 mmHg for 5 min. The retinoprotective effect of Semax at a dose of 7.2 μg/100 g of rat mass, in comparison with Picamilon at a dose of 3 mg/100 g of rat mass, was estimated by laser Doppler flowmetry (LDF) and electroretinography (ERG). Results: The use of Semax led to an increase in retinal perfusion by 62.7%, p < 0.05, in comparison with the group with the model, and by 9.9%, p < 0.05, in comparison with Picamilon; an increase in the b/a coefficient by 31.4% in comparison with the group with the model, p < 0.05, and by 14.6%, p < 0.05 in comparison with Picamilon. Conclusion: The neuroretinoprotective effect of Semax in correction of hypertensive retinal changes in rats may be due to the presence of neuroprotective, neurometabolic, antioxidant and endothelioprotective effects in Semax. Thus, Semax can be a promising agent in hypertensive neuroretinopathy treatment.
Ключевые слова: Semax, hypertensive neuroretinopathy, rats, laser doppler flowmetry, electroretinography
К сожалению, текст статьи доступен только на Английском
Introduction. With long-term, advanced hypertensive disease, the target organs (retina, kidneys, brain) are affected. Hypertension can affect the eyes in several ways, including the development of retinopathy and optic neuropathy. Hypertension is a risk factor for other eye diseases, including occlusion of the central retinal artery (CRA) and its branches, macroaneurysms of the retinal arteries and others [1, 2].
Published data indicate a 3-14% prevalence of hypertensive retinopathy in patients over 40 years of age. The pathogenetic link of hypertensive retinopathy is retinal ischemia, which can lead to the optic nerve atrophy [3, 4, 5]. In 63 % of patients with hypertension, there are manifestations of hypertensive angiopathy. Vascular endothelium is attributed to the most early damaged target organ in hypertension, as well as to the cause of increased blood pressure [6, 7].
Special interest is paid to the development of short-chain peptide drugs for the cytoprotection [8, 9, 10, 11, 12], in particular, neuroprotection [13, 14]. One of the actively studied classes of peptide regulators are melanocortins, which accelerate regeneration in the neuromuscular system, have a protective effect on damage to the central nervous system. Melanocortins have hormonal activity, positive effect on the development of the nervous system, etc. This class of peptides includes an analog of adrenocorticotropic hormone (ACTH)(4-10) with prolonged action Semax. To date, Semax is the only widely used in clinic neurotropic drug developed on the basis of melanocortins. Semax has no hormonal activity and retains a significant part of the spectrum of neurotropic effects of natural melanocortins. The heptapeptide Semax is a synthetic analogue of ACTH(4-10) which exerts marked nootropic and neuroprotective activities [15], protects effectively the brain against ischemic stroke [14]. Semax promotes the survival of neurons during glutamate excitotoxicity [16], protects against the optic nerve atrophy and optic neuritis of inflammatory or toxic-allergic etiology [17].
At present, the spectrum of physiological and pharmacological activity of Semax is actively being studied, because many aspects of the therapeutic effects of this drug remain unknown. Based on literature data, tripeptide Pro-Gly-Pro (PGP) was predominant in a mixture of Semax derivatives in rat blood plasma and brain tissues just 1 h after intraperitoneal administration of Semax [18]. Independent effects of PGP were revealed recently, including an effect on cell culture survival in oxidative stress [19]. The effect of Semax and PGP previously was shown on the expression of genes that encode neurotrophic factors and their receptors in a model of brain ischemia in rats [20]. However, despite the advances described above, the molecular mechanisms underlying the Semax neuroprotective action and the degree of PGP participation in them remain obscure [15].
In cerebral ischemia, Semax showed neuroprotective, neurometabolic and antioxidant effects, and also promotes the synthesis of BDNF and NGF in the brain [13]. It was shown that Semax and PGP increased proliferation of the neuroglia, endothelium, and progenitor cells in the subventricular zone. Semax influences the genes expression associated with the vasodilation of arteries. It was shown that capillary bore dilation was observed as early as 15 min after the administration of Semax [20].
Under transient middle cerebral artery occlusion (tMCAO) conditions, it was found that Semax initiated mRNA expression that counteracted ischaemia-reperfusion (IR). In particular, Semax suppressed inflammatory and activated neurotransmitter genes, whereas the genetic response initiated by IR activates inflammatory and suppresses neurotransmitter genes. It was revealed that significant compensation effects of Semax peptide on inflammatory and neurotransmitter genetic responses after tMCAO, which may account for the neuroprotective action of Semax under IR conditions. Thus, an important feature of Semax is the normalization of mRNA expression patterns that are disturbed during ischaemia [21].
An important feature of Semax is the speed of the onset of the therapeutic effect, the absence of drug dependence and withdrawal syndrome. After intranasal administration, Semax penetrates the blood-brain barrier within a few minutes, and with a single administration, the therapeutic effect lasts up to 24 hours. The prolonged action of Semax is due to its sequential transformation, while most of the properties of the drug are preserved in its fragments EHFPGP and HFPGP. These fragments are quite stable and independently modulate cholinergic neurotransmission and nitric oxide synthesis [13].
Intranasal administration of Semax over the 7-day treatment course resulted in a 25% decrease of infarct volume in the prefrontal cortex and cognitive function recovery over the long term [22]. Recently, it was shown that Semax activates and induces PGC-1α in the penumbral neurons in a model of focal ischemia and exert neuroprotective effects through these mechanisms. The target molecule – the PGC-1α transcriptional coactivator is known for its pleiotropic potentiating effect on neuron viability and functionality. This study was performed in a model of photochemically induced thrombosis of prefrontal cortex blood vessels, mimicking the pathogenesis of acute ischemic stroke. Indeed, on the third day after photochemical-induced thrombosis, signs of degenerative changes in the microcirculatory bed were detected, such as multiple hemorrhages, pronounced vasodilation, and erythrocyte stasis. Secondary post-stroke perfusion disturbances led to a significant progression of the penumbra, while the administration of Semax limited the disturbances of peri-infarction microcirculation observed after photochemical-induced thrombosis [23].
The intramuscular injection of Semax at a dose of 200 μg/kg for 10 days in animals with diabetes did not significantly influence the content of circulating endothelial cells and stable metabolites of NO, but the concentration of von Willebrand factor and endothelin-1 was significantly reduced (by 16.8 and 15.4%, respectively, p < 0.01) as compared to the control [24].
In addition, it was shown that the administration of Semax had antioxidant action and caused the increase in the antioxidant enzymes activity in a model of diabetes mellitus. Under the influence of Semax, the content of ceruloplasmin was lower. The administration of Semax and Sulodexid had antioxidant action, similar to Semax injection only. The decrease in the content of malondialdehyde was 17.8% (p < 0.01), and acylhydroperoxides – by 28.4% (p < 0.01). The drug had a stimulating action on the activity of superoxide dismutase (SOD) (by 32.7% compared to the control, p < 0.01) and catalase – by 23.6% (p > 0.05). The received results show the antioxidant action of Semax in diabetes mellitus and confirm the prospect of their use in the endothelial dysfunction correction as a separate drug or in the combination with some other endothelioprotectors [25].
Studying the new perspective ways of neuroprotection in retinal injuries, in particular, developing in hypertension, is of great interest [26]. Therefore, an important task is to find specific and effective agents for treatment of hypertensive neuroretinopathy. To study the new pharmacological properties of drugs, it is necessary to conduct further studies in vivo [27, 28, 29, 30] on the adequate experimental pathology models [31].
Thus, it is perspective to study the possibility of pharmacological correction of hypertensive retinal changes with Semax in laboratory rats.
The aim of this research is to evaluate the correction possibility of retinal injuries with Semax in a rat model of hypertensive neuroretinopathy.
Materials and Methods
Animals. The experiments were conducted on 40 Wistar rats of average weight 250 g. For the study, the healthy animals were taken, having passed the quarantine. Ethical principles of conducting experiments on laboratory rats were observed in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, CETS No. 123. Manipulations on animals were performed under general anaesthesia with intraperitoneal (i.p.) administration of chloral hydrate solution at a dose of 30 mg/100 g of rat mass.
Design of the Experiment. The experiment included the following groups:
1) a control (with i.p. saline for 28 days) (n = 10);
2) a group with simulated hypertensive neuroretinopathy (HNRP) (n = 10);
3) a group with Semax at a dose of 7.2 μg/100 g in the model of HNRP (n = 10);
4) a group with Picamilon at a dose of 3 mg/100 g in the model of HNRP (n = 10).
HNRP simulation was conducted by daily i.p. administration of N-nitro-L-arginine methyl ester (L-NAME) (Sigma, Germany) at a dose of 1.25 mg/100 g of rat mass in a solution form within 28 days and a single increase in intraocular pressure (IOP) to 110 mmHg by applying mechanical pressure to the anterior chamber of the eye for 5 min on the 26th day of the experiment [26]. The increase in IOP was conducted under general anaesthesia (i.p. chloral hydrate, 30 mg/100 g of rat mass).
For the study, Semax nasal drops, 0.1% (PEPTOGEN Innovative Research and Production Center) were used. In diseases of the optic nerve, Semax is instilled 2-3 drops in each nasal passage 2-3 times/day. The daily dose is 600-900 μg. The course of treatment is 7-10 days [32]. 1 drop of the standard solution contains 50 μg of the active substance, 0.05 ml of the solution. The conversion factor for an adult with a body weight of 70 kg is 39. For a rat weighing 250 g, the conversion factor is 7.0. Thus, the estimated dose (ED) of Semax was calculated:
EDvol = 0.9 • 39 / 70 • 7 = 0,072 (ml/kg/day)
EDm = 0.072 • 50 / 0.05 = 72 (μg/kg/day) = 7.2 (μg/100 g/day)
A solution of nasal drops was administrated into the nasal cavity using a micropipette once a day daily for 7 days, from the 22nd to the 28th days of the experiment.
The administration of Picamilon (a reference drug) at a dose of 3 mg/100 g (Pharmstandard-UfaVITA JSC, Russia) was conducted 60 minutes before L-NAME administration, from the 22nd to the 28th days of the experiment, inclusive. Picamilon was daily administered intragastrically (i.g.). The choice of Picamilon as a reference drug in the hypertensive neuroretinopathy model, the dose and route of its administration to rats is based on its effectiveness in previously conducted experimental studies at the Research Institute of Pharmacology of Living Systems (BelSU) and published data [26].
The effectiveness of the pharmacological correction with Semax and Picamilon was evaluated on the 29th day of the experiment by the b/a coefficient and retinal microcirculation level.
Laser Doppler Flowmetry. 72 hours after the increase in IOP, the retinal perfusion in rats was measured by LDF. LDF is a non-invasive method for assessing the blood flow in tissues. In LDF, a coherent laser beam illuminates the vascular tissue and measures the Doppler shift caused by the movement of red blood cells. The movement of red blood cells causes a shift in the frequency of scattered light, and the walls of the vessels produce static scattering without any shift in the frequency of light and serve as a reference signal. The registration was performed using MP150 production Biopac System, Inc. (Goleta, USA), a computer‐based data acquisition system with the AcqKnowledge 4.2 software, and a TSD‐144 needle‐type sensor (Biopac System, Inc., Goleta, USA). After the rats were anaesthetized, the retinal perfusion was measured at 10 points on the circumference of the eye [4].
Electroretinography. ERG is the electrical response of the retina to light stimulation. A flash of light causes a two-phase negative-positive waveform. Wave a, which occurs at the photoreceptor level, is the initial large negative wave. The b wave that occurs in the inner retina is the next big positive component. The effects of drug therapy can be detected and quantified using ERG [33]. The assessment of retinal functional activity was conducted with a- and b-wave amplitudes. ERG was performed in rats with our previously published method [2, 4]. The ratio of the amplitudes of the b‐ and a‐waves, the b/a coefficient, was calculated.
Statistical Data Processing. The data were checked for type of distribution. In normal distribution, the average value (M) and standard error of the mean (m) were calculated. In abnormal distribution, the median (Me) and the quartile range (QR) were calculated. Between-group differences were analyzed by parametric (t-Student criterion) or non-parametric (Mann-Whitney U-test) methods. The differences were determined at a 0.05 significance level. The statistical analyses were performed using Statistica 10.0 software.
Results.
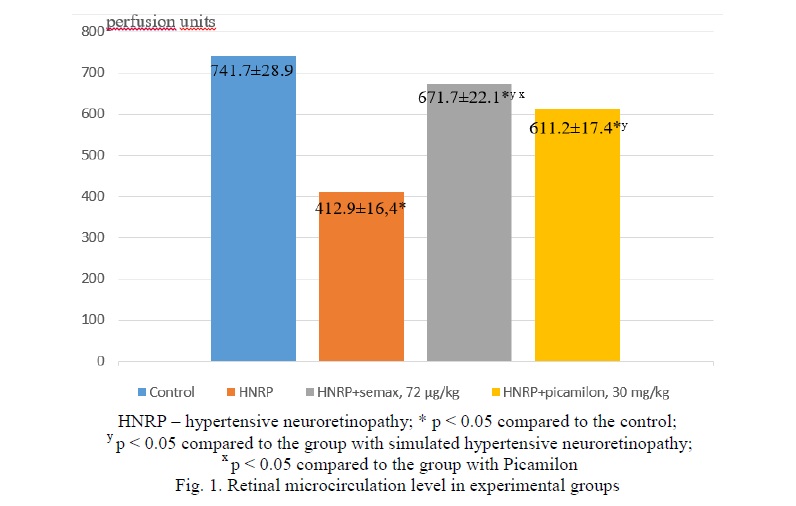
LDF results. Results of retinal microcirculation evaluation are presented in Figure 1. In the group with the HNRP simulation, the retinal perfusion decreased by 44.3% (p < 0.05) in comparison with the control. When correcting HNRP with Semax at a dose of 7.2 µg/100 g, perfusion level differed significantly (by 9.4%, p < 0.05) from the mean value of the control group, increased by 62.7% (p < 0.05) in comparison with the group with HNRP and differed significantly (by 9.9%, p < 0.05) from the mean of the group with Picamilon at a dose of 3 mg/100 g.
When correcting HNRP with Picamilon, the microcirculation level in the retina differed significantly (by 17.6%, p < 0.05) from the mean of the control group, increased by 48.0% (p < 0.05) in comparison with the group with HNRP simulation.
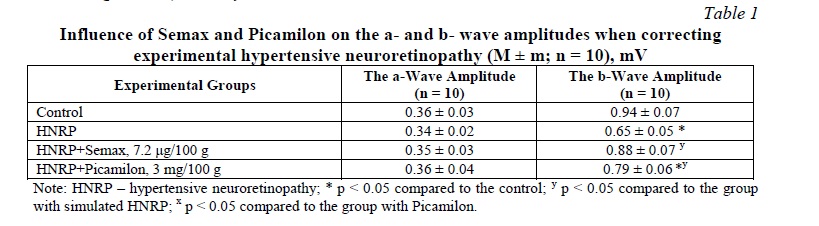
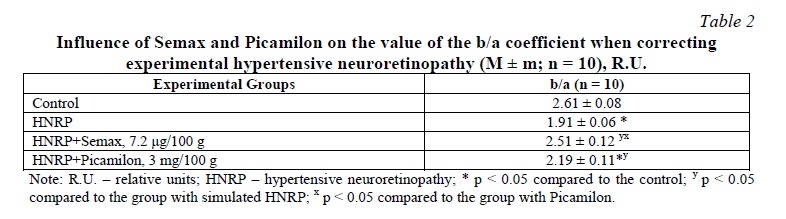
Results of the ERG, and b/a Counting. Theinfluence of Semax and Picamilon on the values of amplitudes of a- and b-waves in groups is presented in Table 1. Further, the b/a coefficient was calculated, the values of which are presented in Table 2. In the group with HNRP simulation, the b/a coefficient decreased by 26.8% in comparison with the control group (p < 0.05). Against the background of Semax, the b/a coefficient increased by 31.4% in comparison with the group with no treatment (p < 0.05), and by 14.6% in comparison with the group with Picamilon (p < 0.05). In the group with Picamilon, the b/a coefficient did not reach the target values, but increased by 14.7% in comparison with the group with no treatment (p < 0.05).
In our opinion, the protective effect of Semax in correction of hypertensive retinal changes in rats may be associated with the presence of neuroprotective, neurometabolic antioxidant and endothelioprotective effects in Semax. Based on the obtained data of the retinal perfusion in the animal groups in the model of hypertensive neuroretinopathy, it follows that a positive effect on the state of the retinal perfusion in descending order has Semax at a dose of 7.2 µg/100 g, then Picamilon at a dose of 3 mg/100 g. Based on the obtained values of the b/a coefficient, it follows that a positive effect on the electrophysiological state of the retina in the correction of hypertensive neuroretinopathy in descending order has Semax at a dose of 7.2 µg/100 g, then Picamilon at a dose of 3 mg/100 g.
It is well known, that the key link in the pathogenesis of endothelial dysfunction is NO deficiency, and the use of drugs that increase NO production has an endothelioprotective effect. Oxidative stress in the retina, which occurs in hypertensive neuroretinopathy, leads to a decrease in NO production, acceleration of its breakdown, and suppression of the expression of endothelial NO synthase (eNOS) [34]. The reasons for the decrease in bioavailability of NO under oxidative stress are an increased level of dimethylarginine formation, which is an endogenous competitive inhibitor of eNOS, as well as a violation of the penetration of L-arginine into endothelial cells under the influence of oxidized low-density lipoproteins [35]. In connection with the above, the study of the retinoprotective effect of Semax as a potential corrector of endothelial dysfunction is of particular interest in the model of hypertensive neuroretinopathy.
It is planned to study endothelioprotective effect of Semax at a dose of 7.2 μg/100 g of rat mass in the retinal vessels in correction of hypertensive retinal changes in rats using immunohistochemistry, namely the influence on eNOS expression.
Conclusion. Thus, when correcting HNRP with Semax at a dose of 7.2 µg/100 g, retinal perfusion increased by 62.7% (p < 0.05) in comparison with the group with no correction and differed significantly (by 9.9%, p < 0.05) from the mean value of the group with Picamilon at a dose of 3 mg/100 g.
In the group with Semax, the b/a coefficient increased by 31.4% in comparison with the group with no correction (p < 0.05), and by 14.6% in comparison with the group with Picamilon (p < 0.05). Therefore, Semax can be a promising agent in the treatment of hypertensive neuroretinopathy.




















Список литературы