АНАЛИЗ АССОРТИМЕНТА РОССИЙСКОГО РЫНКА ЛЕКАРСТВЕННЫХ СРЕДСТВ ДЛЯ ЛЕЧЕНИЯ БЕРЕМЕННЫХ С ОРВИ
Aннотация
Проведен анализ российского рынка лекарственных препаратов разрешенных к применению у беременных заболевших острыми респираторно-вирусными инфекциями.
Ключевые слова: анализ ассортимента лекарственных средств, российский рынок, беременные, острые респираторно-вирусные инфекции
К сожалению, текст статьи доступен только на Английском
Respiratory diseases today are a major global health problem. For example, the incidence of these pathologies in the Russian Federation increased by 12.4% during the period from 2005 to 2011, and the temporary disability has increased and now takes the first place - 28% of all cases. The mortality rate, according to statistics, in 2009 - 2010 was 56.0 cases per 100 thousand people [2].
In the world, the influenza and acute respiratory viral infections (ARVI) cause the main economic damage in the structure of infectious respiratory diseases. Up to 90% of cases is accounted for acute respiratory diseases [1, 7], which is about 30 million cases in Russia annually. However, the actual number of cases often not seeking medical care 1.5-2 times exceeds the official data [8].
All categories of population are susceptible to ARVI, but for some groups of people, they are the most dangerous. The main risk group is represented by pregnant women, whose incidence structure’s leading position is occupied by this pathology [3, 4, 5, 6]. During pregnancy, there is a physiological depression of immunity, so a woman's body becomes weak and susceptible to various colds and infectious diseases [9].
Currently, there is a broad range of medicinal drugs (MD) for the treatment of ARVI, but their application by pregnant women is limited. Clinical trials of the majority of medicinal products (MP) in terms of their safe use during pregnancy are either limited, or have not been carried out yet. Prescription of medicinal treatment is also complicated due to the absence of pharmacological standards and formulary lists of medicinal products for the treatment of pregnant women with acute respiratory viral infection [10].
In connection therewith, it is relevant to study the market of medicinal products used for the treatment of acute respiratory viral infections in pregnant women.
Objective of the research: to study the market of Russian medicinal products used for treatment of acute respiratory viral infection in pregnant women.
Materials and research methods. To conduct the study we used official information directories of medicinal products: Vidal «Medicines in Russia» 2014, «Register of medicinal products of Russia» for 2014-2015; and the «Analyte-Pharmacy» software.
Methods of research: content analysis, structural, comparative, graphic, and segmentation analyses.
Results and discussion.
To archive the set objective, we developed the research concept consisting of three stages: the formation of drug information array; structural analysis of the range of medicinal products; and the development of macroprofile of the Russian pharmaceutical market of medicinal products used in treating ARVI in pregnant women.
During the first stage, we analyzed the official sources of information about medicinal products and based thereon generated the information array of medicinal products for the treatment of ARVI in pregnant women. We revealed that the range includes 503 medicinal drugs, 217 trade names and 67 international non-proprietary names (INN) medicines.
During the second stage of the study, we revealed that the structuring of the range fully on the Anatomical Therapeutic Chemical Classification (ATC) is not possible, and therefore we added to the structure the drugs for ARVI treatment on the International Classification of Diseases, 10th edition (ICD-10): homeopathic medicines, herbal MD and medicinal plant product.
It was found that seven groups (four - on the ATC-3 classification, and three pharmacotherapeutic groups) represent the range of the Russian market of drugs for the treatment of acute respiratory viral infections in pregnant women. Group R «Respiratory system» ranks first in the range structure - 293 medicinal drugs (58.3%). The second is the «Medicinal plant product» - 54 MD (10.7%), and the third is group N «Nervous system» - 50 MD (9.9%). Further, there are herbal drugs - 42 MD (8.3%); group L «Antineoplastic and immunomodulating agents» - 30 MD (6%); homeopathic drugs - 21 MD (4.2%), and group J «Antivirals for systemic use» - 13 MD (2.6%) (Table 2).
Table 2
The range structure of the medicinal drugs used in treating acute viral respiratory infections in pregnant women, presented in the Russian pharmaceutical market
No.
| Classification group | Number of items | |||||||||||||
INN | Trade name | Medicinal preparations | |||||||||||||
Total | incl. domestic | incl. foreign | incl. new | ||||||||||||
Abs. | Share, % | Abs. | Share, % | Abs. | Share, % | Rank | Abs. | Share, % | Abs. | Share, % | Abs. | Iо, % | |||
R Respiratory system | |||||||||||||||
1 | R01 Nasal preparations | 14 | 20.9 | 43 | 19.8 | 108 | 21.5 | II | 52 | 48.1 | 56 | 51.9 | 62 | 57.4 | |
2 | R02 Throat preparations | 25 | 37.3 | 42 | 19.4 | 60 | 11.9 | III | 26 | 43.3 | 34 | 56.7 | 29 | 48.3 | |
3 | R05 Cough and cold preparations | 12 | 17.9 | 50 | 23 | 125 | 24.9 | I | 52 | 41.6 | 73 | 58.4 | 54 | 43.2 | |
Total for group | 51 | 76.1 | 135 | 62.2 | 293 | 58.3 |
| ||||||||
N Nervous system | |||||||||||||||
4 | N02 Analgesics | 6 | 9 | 21 | 9.7 | 50 | 9.9 | V | 35 | 70 | 15 | 30 | 18 | 36 | |
J Antiinfectives for systemic use | |||||||||||||||
5 | J05 Antiviral preparations for systemic use | 3 | 4.5 | 3 | 1.4 | 13 | 2.6 | IX | 8 | 61.5 | 5 | 38.5 | 10 | 76.9 | |
L Antineoplastic and immunomodulating agents | |||||||||||||||
6 | L03 Immunostimulants | 7 | 10.4 | 14 | 6.5 | 30 | 6 | VII | 25 | 83.3 | 5 | 16.7 | 15 | 50 | |
Preparations of other groups | ||||||||||||||
7 | Homeopathic medicines | - | - | 19 | 8.7 | 21 | 4.2 | VIII | 1 | 4.8 | 20 | 95.2 | 6 | 28.6 |
8 | Herbal MD | - | - | 20 | 9.2 | 42 | 8.3 | VI | 19 | 45.2 | 23 | 54.8 | 16 | 38.1 |
9 | Medicinal plant product | - | - | 5 | 2.3 | 54 | 10.7 | IV | 54 | 100 | - | - | 28 | 51.9 |
Total | 67 | 100 | 217 | 100 | 503 | 100 |
| 272 | 55.3 | 231 | 44.7 | 238 | 47.8 | |
Studying the MD used for treatment of ARVI in pregnant women, we excluded categories of drugs such as «contraindicated» and «not recommended». The rest of the MD were ranked and grouped. In the course of study, we divided the entire range into 6 subgroups. The leading position is occupied by a subgroup of drugs, which can be used only after consultation with a doctor or upon doctor’s prescription and evaluation of the potential benefit for the mother and the risk to the fetus. Drugs of this group make a majority - they account for 57.2% (288 MD) of the total number of MD used during pregnancy. The second are medicinal drugs, the use of which is prohibited during the 1st trimester of pregnancy, and their further use is only possible after consulting a doctor and evaluating the risks and benefits - 16.1% (81 MD). The third is a group of «Use with care» drugs – 14.3% (72 MD). Further, there are groups of «Use permitted» - 6% (30 MD), «Use possible» - 5% (25 MD), and «Contraindicated in the 1st trimester of pregnancy» – 1.4% (7 MD) (Fig. 1, Table 3).
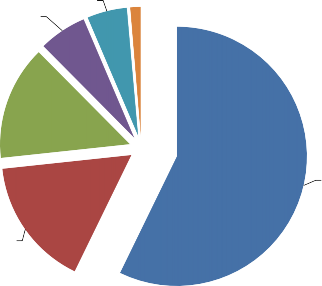
Fig. 1. Segmentation of the MD range by the possibility of using in pregnant women, %
We conducted the structural analysis of the MD range by INN. It was found that the leading position is occupied by a group R «Respiratory system» – 76.1% (51 MD). Further, there are group L preparations «Antineoplastic and immunomodulating agents» – 10.74% (7 MD); group N «Nervous system» - 9% (6 MD), and group J «Antivirals for systemic use» - 4.5% (13 MD) (Table 3). Analysis of the range structure by trade names showed that the leading group is also group R «Respiratory system» - 62.2% (135 MD TN). Further, there are group N «Nervous system» - 9.7% (21 MD), herbal preparations – 9.2% (20 MD), Homeopathic preparations - 8.7% (19 MD), «Antineoplastic and immunomodulating agents» – 6.5% (14 MD), «Medicinal plant product» - 2.3% (5 MD), and «Antivirals for systemic use» – -1.4% (3 MD).
During segmentation analysis based on production, we found the predominance of the domestic drugs - 54.1% (272 MD) over foreign - 45.9% (231 MD).
Analysis of offers for drugs of foreign manufacturing countries showed that there are offers of total 25 foreign countries registered. The leading positions among them belong to Germany (30.3%), France (7.4%), and India (6.9%). They are followed by Great Britain (6.1%), Switzerland (5.6%), Italy (5.2%), Slovenia (4.3%), the USA (3.5%), Czech Republic (3.5%), and Croatia (3%). The rest of the foreign manufacturing countries are grouped as «Others» and account for 24.2% (56 MD).
The next stage involved segmentation of the drugs range by their composition. It was found that of the dominant part in the structure belongs to single component preparations - 54.9%, while the combined MD account for 45.1%.
The MD segmentation by type of dosage form (DF) showed that four groups - liquid, solid, soft, and gaseous, represent the range. The liquid MD prevail and account for 50%. The leading position among them belongs to sprays that are 31.3% (70 MD). Further, there are drops - 25.9% (58 MD), syrups - 24.6% (55 MD,) solutions - 16.5% (37 MD), suspensions - 1.3% (3 MD), and emulsions - 0.4% (1 MD).
The solid MD are the second - 43.4%. The leading position among them belongs to tablets - 65.1% (127 MD). Further, there are powders - 9.2% (18 MD), suppositories - 8.2% (16 MD), capsules - 6.2% (12 MD), lyophilizates - 4.6% (9 MD), pastilles - 3.1 % (6 MD), granules – 2.1% (4 MD), and pellets - 1.5% (3 MD).
Soft and gaseous MD are the third - each of 3.3% (15 MD each). The leading position among soft MD belongs to ointment – 86.6% (13 MD), then gels and creams – each 6.7% (1 MD each). Gaseous MD are represented by aerosols - 3.3% (15 MD).
We analyzed the dynamics of MD registration and revealed that the renewal index in all groups of studied preparations took on a value of 28.6% to 76.9% during 2009-2014 in the Russian market, and was 47.8% on average.
It was found that the renewal index was maximum for the group J05 «Antiviral preparations for systemic use» - 76.9% (10 MD). The second is a subgroup R01 «Nasal preparations» - 57.4% (62 MD). Further, there are medicinal plant product - 51.9% (28 MD), L03 «Immunostimulators» - 50% (15 MD), R02 «Throat preparations» - 48.3% (29 MD), R05 «Cough and cold preparations» - 43.2% (54 LP), herbal drugs - 38.1% (16 LP), N02 «Analgesics» - 36% (18 LP), and homeopathic medicines - 28.6% (6 MD).
Based on these findings, we formed the macroprofile of the Russian pharmaceutical market of drugs used for the treatment of acute respiratory viral infection in pregnant women, which is represented mainly by a group R «Respiratory system» (58.3%), where the leader by the absolute number of the MD is a group R05 «Cough and cold preparations» (24.9%). The domestic medicines prevail in terms of production (54.1%). The single component drugs dominate in the general structure of the Russian market (54.9%). 50% of the analyzed range is accounted for liquid MD, mostly in the form of sprays - 31.3%. The average renewal index is 47.8% (Fig. 2).
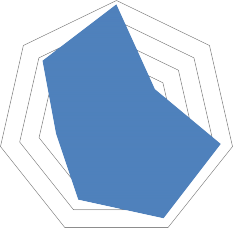
R Дыхательная система – RRespiratorysystem. R05 Препараты, применяемые при кашле и простудных заболеваниях – R05 Coughandcoldpreparations. Отечественные ЛП – DomesticMD
Монопрепараты – Mono-preparation. Жидкие ЛФ – LiquidDF
Спреи – SpraysИндекс обновления – Renewalindex
Fig. 2. The macroprofile of the Russian pharmaceutical market of medicinal drugs used in treating acute viral respiratory infections in pregnant women, %
Thus, the analysis of the Russian pharmaceutical market revealed a significant number of drugs used for the treatment of ARVI. However, the range of drugs permitted or possible for use in pregnant women is only 11% of total range of drugs, which requires, in our opinion, the development of government decisions on the introduction of drugs with a high safety threshold possible for use in pregnant women on the Russian pharmaceutical market.




















Список литературы
Список использованной литературы появится позже.