ОПТИМИЗАЦИЯ МЕТОДОВ КОНТРОЛЯ КАЧЕСТВА ЛЕКАРСТВЕННЫХ ПРЕПАРАТОВ СТЕРОИДНОЙ СТРУКТУРЫ НА ПРИМЕРЕ ГЛЮКОКОРТИКОСТЕРОИДОВ
Aннотация
Предложен оригинальный метод анализа кортикостероидов с использованием обращённо-фазной высокоэффективной жидкостной хромато-графии. В качестве элюирующей системы кортикостероидов подобрана смесь 1%-ного водного раствора кислоты муравьиной – спирт этиловый в соотноше-ниях 10:90. Такое соотношение растворителей в подвижной фазе позволяет кон-тролировать скорость перемещения кортикостероидов по колонке. Поскольку кортикостероиды хорошо растворимы в спирте, то использование в подвижной фазе 90% этого растворителя позволило получить оптимальное время удержи-вания данных соединений. Также важным достоинством данной системы явля-ется то, что проведена замена дорогостоящих и токсичных ацетонитрила и ме-танола, традиционно используемых в мировой практике в методе обращённо-фазной ВЭЖХ на нетоксичный и доступный спирт этиловый. Качество получа-емых результатов хроматографирования не изменилось. Поскольку кортикосте-роиды имеют характерные полосы поглощения в УФ- и видимой областях спек-тра, то для регистрации результатов использован диодно-матричный детектор. Время анализа составило не более 5 минут, что характеризует данный хромато-графический процесс как весьма экспрессный.
Ключевые слова: глюкокортикостероиды, высокоэффективная жидкостная хроматография, критерии пригодности хроматографической системы
К сожалению, текст статьи доступен только на Английском
Introduction. Steroid hormones are one of the most important groups of drugs in the modern pharmaceutical market. The most important feature of this group of drugs is the breadth of pharmacological effects and especially the impact on all metabolic processes of the body. However, steroid compounds are a quite diverse group of compounds in terms of their points of application. There are sex hormones and hormones of the adrenal cortex - corticosteroids. The latter group of hormones features pronounced influence on the exchange of various substrates in the body, primarily carbohydrates. Furthermore, their characteristic property such as regulation of immune processes in the body determines their breadth of use in different clinical forms, including the most severe autoimmune diseases. None of other pharmacological group has similar vector of action at the present time, so these drugs are virtually indispensable in all fields of medicine [5].
Given the uniqueness of corticosteroid hormones in medical practice, the methods of their quality analysis are highly essential, because sound quality of these compounds depends entirely on their standardization.
Currently, a significant amount of analysis methods for corticosteroids has been developed, due to their long-time application in medicine. Corticosteroids are mainly analyzed by instrumental methods of analysis, due to their structural features.
Spectral analysis methods are most widely used to study these compounds. The presence of common chromophore fragment - a ketodienoic group - ensures their absorption in the UV-area of the spectrum. Therefore, UV spectroscopy is one of the most important methods of analysis of corticosteroids, which is used for both identification, and their quantification.
IR spectroscopy is suitable for the identification of steroids and the establishment of structural features in their formula because it can detect the presence of specific functional groups and structural features of the molecules.
The steroid compounds have a hard skeleton - a sterane core and conjugated bonds, so a fluorometer is also required for their analysis. Fluorometric method is quite sensitive, therefore indispensable for detecting the trace amounts of corticosteroids, in particular, in the biopharmaceutical analysis [2].
Chromatographic techniques are the most important for the analysis of corticosteroids. There is a large number of analysis layouts developed, corticosteroid mobile phases chosen in various objects with the use of planar chromatographic variants such as paper and thin-layer ones.
An essential variant of corticosteroid chromatography is the method of gas-liquid chromatography. Due to thermal stability of these compounds and their ability to derivate into volatile forms, this method has widespread application in their analysis.
HPLC-method has found wide application for the standardization of corticosteroids, especially in the direct-phase variant. This is due to mild conditions of HPLC, and a large number of structural information obtained from the analysis results.
However, each of the said methods of corticosteroid analysis has its own significant drawbacks. In particular, spectral methods can only provide information on the structure of the chromophore or the presence of functional groups, but no information about the purity of analyzed objects.
Methods paper and thin-layer chromatography are characterized by an inadequate sensitivity, objectivity and give little information about the analyte.
Although gas-liquid chromatography is characterized by sensitivity and objectivity, it, however, requires complex sample preparation and conditions of analysis.
HPLC has become one of the most valuable methods for separation and determination of corticosteroids. One of such possible causes for displacing the gas chromatography with HPLC in the corticosteroid analysis is the thermal sensitivity of a side chain C-17. In addition, HPLC allows determining all corticosteroids with quite high sensitivity by their intense UV-absorption band in. The high sensitivity of HPLC allows addressing almost all the problems that can arise for analysts in the field of pharmaceuticals and biomedicine [1].
Corticosteroids can form isomers due to the presence of asymmetric carbon atoms in their structure, so the advantage of HPLC method lies in the possibility of separating these isomers. As for the chromatographic separation systems, both adsorption chromatographic and distribution reverse-phase systems have been widely used. The UV-detectors are almost exclusively used for the chromatograms analysis. The absorbance is usually measured on a spectrophotometric ultraviolet detector at 240 nm [2, 7].
Given the above, the development of new approaches to the analysis of corticosteroids is a topical problem.
Objective of the research was to develop the optimum methods for the analysis of corticosteroids with the use of reversed-phase high performance liquid chromatography (HPLC).
Materials and methods. The objects of this study were corticosteroid-containing medications such as «Dexamethasone» eyedrops, «Triamcinolone» tablets, and «Prednisolone» injections.
Chromatographic studies were conducted on the chromatographic apparatus by Agilent Technologies 1200 Infinity (USA) with autosampler Agilent 1200, vacuum micro-degasifier, gradient pump, and thermostat of the same series. Electronic absorption spectra were recorded with a spectrophotometric diode-array detector Agilent 1200 (a wavelength range of 190 to 950 nm, a cuvette with a path length of 10 mm and volume of 13 μl), the scanning pitch - 2 nm.
Registration and processing of spectral data and chromatograms was performed with the use of Agilent Chem Station software.
The tests were carried out with a steel chromatographic column Ascentis express C182.7 μm × 100 mm × 4.6 mm.
The mobile phases were prepared with the use of the following solvents: ultrapure water (liquid chromatography), ethyl alcohol (acc. GOST R 51652), and formic acid.
Column efficiency was determined by calculating the number of theoretical plates N. As an optimal column efficiency criterion the value of not less than 5000 was used [4].
The number of theoretical plates was calculated by formula 1:
where -the retention time of the analyte, mm;
µ- peak width at the baseline, mm.
The main criterion for assessing the adequate separation of adjacent peaks was the separation factor Rs, which shall not be less than 1.5, subject to the European Pharmacopoeia [4]. In this case, the peaks must be separated in the baseline.
Peak separation coefficient Rs was calculated by formula 2:
where - distance between the tops of two adjacent peaks, mm;
- width at half peak height of the two components, mm.
The shape of the chromatographic peak, which characterizes the overload of chromatographic column, was determined by calculating the peak asymmetry coefficient (Tf) by formula 3:

where µ0.05 – peak width at a height of 5.0% of the baseline, mm;
ƒ – distance from the peak base at a height of 5.0% of the baseline to the perpendicular drawn from its top, mm;
The optimum value of the asymmetry coefficient Тf was the one less than 2.
Separation was carried out under the following conditions:
mobile phase: 1.0% formic acid aqueous solution - ethyl alcohol (10 : 90);
mobile phase rate - 0.5 ml/min;
column temperature +35оС;
sample injection volume 1μl.
The investigated corticosteroids contain in the ring A a chromophoric fragment represented by ketodienoic group, therefore absorb optical electromagnetic radiation in the ultraviolet region. Maximum absorption of these compounds is observed in the wavelength range of 238 - 242 nm. Therefore, the UV-spectrophotometry is used for the qualitative and quantitative analysis of corticosteroids and their analogs.
A diode-array detection at a wavelength λ = 240 nm.
Results and discussion. Polkortolone chromatogram is shown in Figure 1.
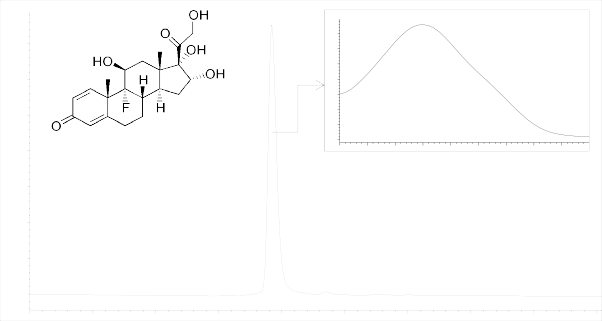
Figure 1-Polkortolone chromatogram
Applicability of the applied chromatographic system was confirmed by calculating the suitability criteria.
The results are shown in Table 1.
Table 1
Indicators of chromatographic system suitability for determining Triamcinolone in tablets
tR | S | N | HETP | Tf | Wb |
1.920 | 376.6 | 10184.81 | 9.8×10-4 | 0.688 | 0.0761 |
tR - absolute retention time, S - area of the peak, N - number of theoretical plates, HETP - height equivalent to theoretical plate, Tf - asymmetry coefficient, Wb - he peak width on the baseline
The suitability criteria in the Table 1 give evidence of the suitability of the applied chromatographic system, because the number of theoretical plates was >5000 and the asymmetry factor was less than 2.
Dexamethasone drug was separated under conditions similar to those of Triamcinolone. Chromatogram is shown in Figure 2.
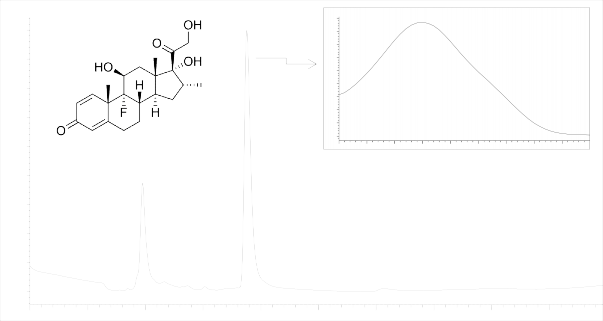
Figure 2- Dexamethasone chromatogram
The suitability criteria of the applied chromatographic system are shown in the Table 2.
Table 2
Indicators of chromatographic system suitability for determining Dexamethasone in eye drops
tR | S | N | HETP | Tf | Wb |
3.758 | 184.9 | 14438.39 | 6.9×10-4 | 0.519 | 0.1251 |
tR - absolute retention time, S - area of the peak, N - number of theoretical plates, HETP - height equivalent to theoretical plate, Tf - asymmetry coefficient, Wb - he peak width on the baseline
Thus, the suitability criteria in Table 2 demonstrate that the applied chromatographic system is suitable for determination of Dexamethasone.
Moreover, the use of the said chromatographic system allowed us to detect the Dexamethasone isomer in the chromatogram - a peak with a retention time of 1.951.
Thus, the chromatographic system ensures determination of both the authenticity of the product «Dexamethasone», and its purity, as can be seen from Figure 2.
Prednisolone chromatogram is shown in Figure 3.
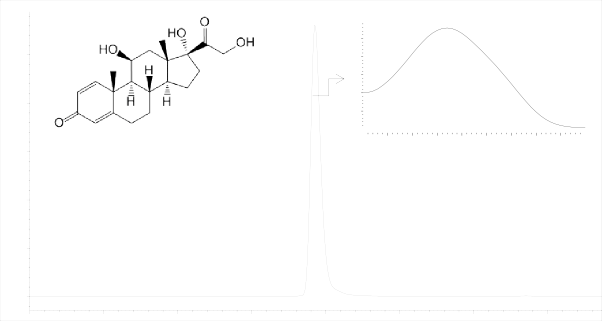
Figure 3- Prednisolone chromatogram
Table 3
Indicators of chromatographic system suitability for determining Prednisolone in the injectable drugs
tR | S | N | HETP | Tf | Wb |
1.927 | 2739.2 | 10562.35 | 9.4×10-4 | 0.677 | 0.075 |
tR - absolute retention time, S - area of the peak, N - number of theoretical plates, HETP - height equivalent to theoretical plate, Tf - asymmetry coefficient, Wb - he peak width on the baseline
The suitability criteria in Table 3 demonstrate that the applied chromatographic system is suitable for determination of Prednisolone.
Summary. As a result of the conducted studies, an original method for corticosteroid analysis has been suggested. In this case, we used a reverse-phase chromatography. Corticosteroids are characterized as low-polarity compounds and therefore can be actively captured by nonpolar stationary phase. However, a mixture of 1% aqueous formic acid and ethyl alcohol at a ratio of 10 to 90 was taken as an eluting system for Dexamethasone, Prednisolone, and Triamcinolone. This ratio of solvents in the mobile phase ensures control of the rate of movement of corticosteroids in the column. Since corticosteroids are freely soluble in alcohol, using 90% of this solvent in the mobile phase ensured optimum retention time of these compounds that did not exceed 5 minutes. Another important advantage of this system is that the expensive and toxic acetonitrile and methanol, commonly used in the world practice in the reversed-phase HPLC were replaced with non-toxic and available ethyl alcohol [3, 6]. Furthermore, the quality of the chromatographic results remained the same. To record the analysis results, we used the diode-array detector that records the electronic absorption spectra, which is an objective feature in the drug data analysis, due to characteristic UV and visible spectrum absorption bands of the corticosteroids. Analysis time was maximum 5 minutes, which characterizes the chromatographic process as express enough.




















Список литературы
Список использованной литературы появится позже.