Estrogens and uterine fibroids: an integrated view
Aннотация
Background: Uterine fibroids or uterine leiomyomata (UL) are common benign tumors of the uterine myometrium affecting a significant proportion of women at reproductive age. UL is a disease with complex etiology determined by many genetic and environmental factors. Estrogen is widely acknowledged as one of the main factors contributing to the risk and pathogenesis of UL. The aim of the study: To summarize available literature data about the estrogen-related environmental factors, genes and metabolic pathways, which may play a role in the disease. Materials and methods: The PubMed, Scopus, and Web of Science literature databases were searched for relevant articles using such keywords as “uterine fibroids”, “uterine leiomyoma”, “estrogen”, “gene”, “association”, “expression”, “epigenetic” in various combinations. Results: Estrogen contributes to the risk and pathophysiology of UL in multiple ways. Apart from the well-known effect of estrogen on expression of many genes mediated by estrogen receptors, it increases sensitivity of myometrium to progesterone and thus influences expression of the progesterone-controlled genes. On the other hand, the data about association of various estrogen-related genes with UL are largely inconsistent and inconclusive. Conclusion: The observed heterogeneity of UL apparently results from the diversity of mechanisms contributing to the disease. This makes identification of the causative genetic variants challenging and prompts for further studies of this problem.
К сожалению, текст статьи доступен только на Английском
Introduction. In eutherian mammals, the uterus facilitates the development of the embryo and the fetus and is necessary for reproduction. The uterus is derived from the Mullerian embryonic ducts and is divided into the inner endometrium and the outer myometrium. The inner endometrium is composed of luminal epithelium, glandular epithelium and endometrial stroma, while the myometrium is formed by smooth muscles. The most common benign pelvic neoplasms that occur in the myometrium smooth muscle layer of the uterus with a prevalence rate of 20-40% in women within their fertile years are uterine fibroids also known as uterine leiomyomata (UL). They are surprisingly very common; approximately 70% of women may develop these fibroids before menopause and 15 to 30% of these women experience serious symptoms [1]. The symptoms of this disease may include excessive menstrual bleeding, abdominal pain, pregnancy complications, pelvic pain, dysmenorrhea, menorrhagia, anemia, urinary incontinence, preterm labor, and infertility in certain instances [2]. UF bear a significant economic burden: the annual direct and indirect costs for the USA alone were estimated at $5.9-34.4 billion [3]. UL is a complex disease and like other multifactorial diseases is determined by many environmental and genetic factors. There is ample evidence that female sex hormones, particularly estrogen, play an important role in pathogenesis of the disease [4]. This review considers estrogen-related factors and mechanisms, which may be involved in the development of UL.
Estrogens and risk for UL
Estrogen has been implicated in pathophysiology of UL due to its pronounced effect on expression of various genes involved in control of cell growth and apoptosis [5, 6]. The elevated level of estrogen in UL patients may result from two sources. The first is estrogen in the bloodstream, while the second is estrogen synthesized in the fibroid tissue through conversion of androgens by aromatase [7, 8]. The latter seems to be particularly important for development of UL as the expression of aromatase was not observed in healthy myometrium [7]. Moreover, this source of estrogen is sufficient for maintaining the growth of the fibroid tissue even without supply of estrogen from other sources [8].
In addition to endogenous estrogen, another risk factor for UL is exposure to various exogenous estrogen-like chemicals. In addition to mimicking effect of natural estrogens, exogenous estrogens may bind to respective hormone receptors and detrimentally affect hormone synthesis and metabolism. One of the common consequences of the exogenous estrogen-like chemicals exposure is precocious puberty [9]. Prenatal exposure to diethylstilbestrol (DES), a synthetic non-steroid estrogen analog, was reported to promote hyper responsiveness to normal levels of estrogen hormone and confer a higher risk of UL [10, 11]. DES was the first synthetic estrogen developed in 1930s and prescribed to pregnant women to prevent miscarriage and to relieve morning sickness. Daughters of DES exposed moms developed adenocarcinomas, uterine structural variation, and were at higher risk of premature labor, ectopic pregnancy, and UL [12, 13]. In addition to DES, environmental phenolic estrogens such as BPA, octylphenol and nonylphenol can be involved in the UL pathogenesis. BPA is a synthetic estrogen used for manufacturing plastic goods and resins that are widely used in food packaging.
Estrogen-regulated gene expression in UL
Estrogen contributes to the growth of UL through several mechanisms. On the one hand, it increases responsiveness of the cell to progesterone [14], which in turn up-regulates the expression of proliferating cell nuclear antigen (PCNA) and epidermal growth factor (EGF) [15]. On the other hand, estrogen affects the expression of various growth factors and apoptosis-related genes. Importantly, this effect can be either inducing or repressing. Specifically, increased expression on the UL tissue was reported for several estrogen-regulated genes, such as type I and III collagen, connexin 43 gap junction protein, parathyroid hormone‐related peptide, IGF‐I and its receptor, and progesterone receptor (PR) [5]. Similar effect was documented for vascular endothelial growth factor (VEGF), a gene contributing to angiogenesis [16], insulin-like growth factor-I (IGF-I) and other genes potentially involved in the IGF-I signaling pathway [17, 18], genes involved in IGF-IR/MAPK signaling [18], and several growth factors [19, 20]. Contrastingly, estrogen reduced levels of actin-A and myostatin mRNA levels in the human myometrium [21], two growth factors, which inhibit myometrial cell growth.
Since UL is an estrogen-dependent disease, two primary estrogen receptor genes (ESR1 and ESR2) have been among the main targets of gene expression studies of UL. Wang et al. [22] determined that the level of the ESR1 expression was elevated in the UL cells as compared to the surrounding normal myometrium, and so was it as compared to ESR2. 1,25-dihydroxyvitamin D3 significantly downregulated the expression of ESR1 and two progesterone receptors [23]. Similar effect on the ESR1 expression was documented after treatment of UL with a gonadotropin-releasing hormone analogue (GnRHa) [22]. The responsiveness of the UL myometrium to estrogen was suggested to be determined by the ratio of the ESR1 to ESR2 expression; it was higher in the cells with the predominant ESR1 expression [24].
The integrated analysis of several UL microarray studies determined twelve estrogen-regulated genes in the ovariectomized rat model treated with estrogen [25]. These genes belong to various metabolic pathways contributing to cell survival and tumor growth. Specifically, estrogen upregulated the anti-apoptotic PCP4 gene and inhibited the expression of growth inhibitory receptors PTGER3 and TGFBR2. Furthermore, estrogen might counteract PPARγ signaling, which is thought to inhibit fibroid growth and survival [25].
One more mechanism of the estrogen stimulating effect on the UL growth was described by Luo et al. [26] who determined that estrogen activated stromal fibroblasts, which, in turn, promoted proliferation of fibroid cells.
Association of estrogen-related genes with UL
Given the acknowledged link between estrogen and UL, attempts have been made to determine association between estrogen metabolism-related genes and UL. However, the number of the respective studies is limited. Their results are summarized in Table 1.
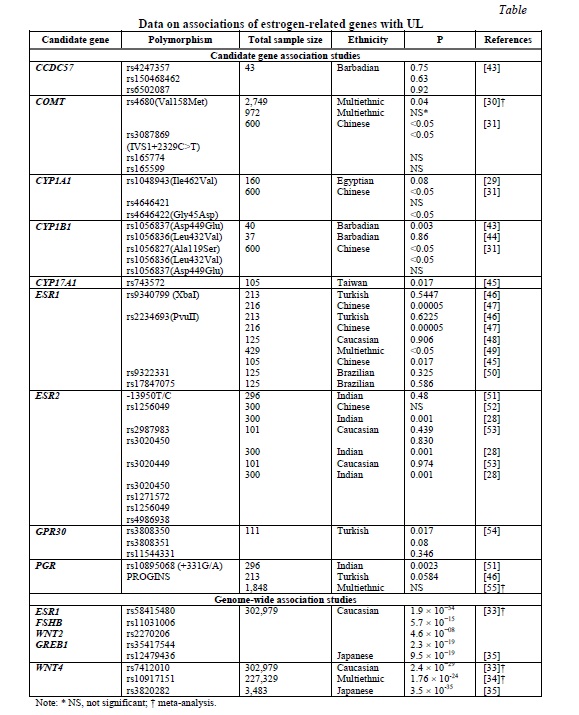
The most commonly analyzed genes were COMT, ESR1, and ESR2. Allele C of the ESR1 PvuII polymorphism was reported to confer approximately a two-fold higher risk for UL [27]. The strong association of five ESR2 gene polymorphisms with UL was documented in the Indian population [28]. Two common polymorphisms of the COMT gene, IVS1+2329C>T and Val158Met, Ile462Val and Gly45Asp loci in CYP1A1 and Ala119Ser locus of CYP1B1 were also suggested as risk factors for UL [29-31]. However, the results of the candidate genes association studies are largely inconclusive and quite limited, so that further more extensive studies are necessary.
The earlier GWAS meta-analysis by Rafnar et al. [32] suggested at least two main genetic pathways contributing to the development of UL: tumorigenesis- and hormone-related, respectively. The GWAS meta-analysis of 35,474 cases and 267,505 controls of European ancestry identified eight novel and confirmed 21 previously reported loci for UL [33]. Among these, several loci were related to estrogen and progesterone signaling, namely ESR1, FSHB, GREB1, WNT2, and WNT4 (Table 1). A transethnic GWAS meta-analysis of Europeans and African-Americans in the sample of 21,804 cases and 205,525 controls supported the previous finding for WNT4[34]. Finally, the most recent GWAS of the Japanese population confirmed GREB1 and WNT4 as candidate genes for UL in this ethnicity [35] (Table 1). However, the results of the GWAS were somewhat inconsistent, which suggests that the genetic basis of the disease is apparently more complex and involve other genes and intergenic interactions not determined by the analyses utilized.
Obesity, estrogen, and risk of UL
Obesity is one of the phenotypes associated with UL. BMI, waist circumference, hip circumference, waist-height ratio, body fat content and body fat percentage are all positively correlated with the development of uterine fibroids [36]. Visceral fat was shown to act as hormone-active tissues, thus by increasing the production of inflammatory mediators, can ultimately lead to UL. The extra-peritoneal fat thickness independently correlates with the development of uterine fibroids (P<0.0001) [37]. The results of this study suggested that the visceral fat area (VFA) was a risk factor for UL. This risk elevated with the increase in the VFA, which in turn correlated with the UL size even though the correlation was relatively weak [37].
A recent meta-analysis further confirmed an increased risk for UL in women with a higher body mass index (BMI) [38]. The observed effect of obesity is apparently explained by the role of the fat tissue in the production of estrone and estradiol from the androgen precursors and the respective increase in the level of circulating estrogen [39]. On the other hand, some data suggested that the effect of higher estrogen level might be opposite for a risk of incident and recurrent UL: it increases the former and decreases the latter [40].
Estrogen-related epigenetic factors
Various epigenetic mechanisms have been implicated in the development of UL [41]. A genome-wide methylation analysis identified 478 genes, which were hypomethylated and 1,014, which were hypermethylated in UL as compared to the surrounding myometrium [42]. Among these, 22 genes, which manifested both aberrant methylation and expression, possessed the consensus sequences of the ER response element and thus were targeted by estrogen. These estrogen-regulated genes contribute to various processes important for pathophysiology of UL, such as extracellular matrix expansion and apoptosis.
Conclusion. Estrogen influences a risk and pathogenesis of UL through multiple mechanisms. This contributes to the heterogeneity of this neoplasm and makes identification of the causative genetic variants challenging.
Financial support
No financial support has been provided for this work.
Conflict of interests
The authors have no conflict of interest to declare.





















Список литературы
1. Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. British Journal of Obstetrics and Gynaecology. 2017;124(10):1501-12. DOI: https://doi.org/10.1111/1471-0528.14640
2. Cook H, Ezzati M, Segars JH, et al. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62(3):225-36.
3. Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. American Journal of Obstetrics and Gynecology. 2012;206(3):211.e1-9. DOI: https://doi.org/10.1016/j.ajog.2011.12.002
4. Reis FM, Bloise E, Ortiga-Carvalho TM. Hormones and pathogenesis of uterine fibroids. Best Practice and Research in Clinical Obstetrics and Gynaecology. 2016;34:13-24. DOI: https://doi.org/10.1016/j.bpobgyn.2015.11.015
5. Andersen J, DyReyes VM, Barbieri RL, et al. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. Journal of the Society for Gynecologic Investigation. 1995;2(3):542-51. DOI: https://doi.org/10.1016/1071-5576(94)00053-4
6. Maruo T, Ohara N, Wang J, et al. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Human Reproduction Update. 2004;10(3):207-20. DOI: https://doi.org/10.1093/humupd/dmh019
7. Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. Journal of Clinical Endocrinology and Metabolism. 1994;78(3):736-43. DOI: https://doi.org/10.1210/jcem.78.3.8126151
8. Sumitani H, Shozu M, Segawa T, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141(10):3852-61. DOI: https://doi.org/10.1210/endo.141.10.7719
9. Bulus AD, Asci A, Erkekoglu P, et al. The evaluation of possible role of endocrine disruptors in central and peripheral precocious puberty. Toxicology Mechanisms and Methods. 2016;26(7):493-500. DOI: https://doi.org/10.3109/15376516.2016.1158894
10. D'Aloisio AA, Baird DD, DeRoo LA, et al. Early-life exposures and early-onset uterine leiomyomata in black women in the Sister Study. Environmental Health Perspectives. 2012;120(3):406-12. DOI: https://doi.org/10.1289/ehp.1103620
11. Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Medical Science Monitor. 2009;15(6):RA137-45.
12. Patel SA, Sunde J. Primary non-clear-cell adenocarcinoma of the vagina in a diethylstilbestrol exposed woman. Military Medicine. 2014;179(4):e461-2. DOI: https://doi.org/10.7205/MILMED-D-13-00316
13. Mahalingaiah S, Hart JE, Wise LA, et al. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses' Health Study II. American Journal of Epidemiology. 2014;179(2):186-91. DOI: https://doi.org/10.1093/aje/kwt250
14. Ishikawa H, Ishi K, Serna VA, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-42. DOI: https://doi.org/10.1210/en.2009-1225
15. Shimomura Y, Matsuo H, Samoto T, et al. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. Journal of Clinical Endocrinology and Metabolism. 1998;83(6):2192-8. DOI: https://doi.org/10.1210/jcem.83.6.4879
16. Hyder SM, Huang JC, Nawaz Z, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environmental Health Perspectives. 2000;108 Suppl 5:785-90. DOI: https://doi.org/10.1289/ehp.00108s5785
17. Swartz CD, Afshari CA, Yu L, et al. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Molecular Human Reproduction. 2005;11(6):441-50. DOI: https://doi.org/10.1093/molehr/gah174
18. Yu L, Moore AB, Castro L, et al. Estrogen regulates MAPK-related genes through genomic and nongenomic interactions between IGF-I receptor tyrosine kinase and estrogen receptor-alpha signaling pathways in human uterine leiomyoma cells. Journal of Signal Transduction. 2012;2012:204236. DOI: https://doi.org/10.1155/2012/204236
19. Ciarmela P, Islam MS, Reis FM, et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Human Reproduction Update. 2011;17(6):772-90. DOI: https://doi.org/10.1093/humupd/dmr031
20. Barbarisi A, Petillo O, Di Lieto A, et al. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. Journal of Cellular Physiology. 2001;186(3):414-24. DOI: https://doi.org/10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E
21. Ciarmela P, Bloise E, Gray PC, et al. Activin-A and myostatin response and steroid regulation in human myometrium: disruption of their signalling in uterine fibroid. Journal of Clinical Endocrinology and Metabolism. 2011;96(3):755-65. DOI: https://doi.org/10.1210/jc.2010-0501
22. Wang H, Wu X, Englund K, et al. Different expression of estrogen receptors alpha and beta in human myometrium and leiomyoma during the proliferative phase of the menstrual cycle and after GnRHa treatment. Gynecological Endocrinology. 2001;15(6):443-52.
23. Al-Hendy A, Diamond MP, El-Sohemy A, et al. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. Journal of Clinical Endocrinology and Metabolism. 2015;100(4):E572-82. DOI: https://doi.org/10.1210/jc.2014-4011
24. Bakas P, Liapis A, Vlahopoulos S, et al. Estrogen receptor alpha and beta in uterine fibroids: a basis for altered estrogen responsiveness. Fertility and Sterility. 2008;90(5):1878-85. DOI: https://doi.org/10.1016/j.fertnstert.2007.09.019
25. Wei T, Geiser AG, Qian HR, et al. DNA microarray data integration by ortholog gene analysis reveals potential molecular mechanisms of estrogen-dependent growth of human uterine fibroids. BMC Women's Health. 2007;7:5. DOI: https://doi.org/10.1186/1472-6874-7-5
26. Luo N, Guan Q, Zheng L, et al. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Translational Research. 2014;163(3):232-41. DOI: https://doi.org/10.1016/j.trsl.2013.11.008
27. Govindan S, Shaik NA, Vedicherla B, et al. Estrogen receptor-alpha gene (T/C) Pvu II polymorphism in endometriosis and uterine fibroids. Disease Markers. 2009;26(4):149-54. DOI: https://doi.org/10.3233/dma-2009-0625
28. Bharathi C, Anupama D, Pratibha N, et al. Impact of genetic variants in estrogen receptor-beta gene in the etiology of uterine leiomyomas. Journal of Reproduction and Infertility. 2019;20(3):151-60.
29. El-Shennawy GA, Elbialy AA, Isamil AE, et al. Is genetic polymorphism of ER-alpha, CYP1A1, and CYP1B1 a risk factor for uterine leiomyoma? Archives of Gynecology and Obstetrics. 2011;283(6):1313-8. DOI: https://doi.org/10.1007/s00404-010-1550-x
30. Feng Y, Zhao X, Zhou C, et al. The associations between the Val158Met in the catechol-O-methyltransferase (COMT) gene and the risk of uterine leiomyoma (ULM). Gene. 2013;529(2):296-9. DOI: https://doi.org/10.1016/j.gene.2013.07.019
31. Shen Y, Xu Q, Ren M, et al. Role of single nucleotide polymorphisms in estrogen-metabolizing enzymes and susceptibility to uterine leiomyoma in Han Chinese: a case-control study. Journal of Obstetrics and Gynaecology Research. 2014;40(4):1077-84. DOI: https://doi.org/10.1111/jog.12275
32. Rafnar T, Gunnarsson B, Stefansson OA, et al. Variants associating with uterine leiomyoma highlight genetic background shared by various cancers and hormone-related traits. Nature Communications. 2018;9(1):3636. DOI: https://doi.org/10.1038/s41467-018-05428-6
33. Gallagher CS, Makinen N, Harris HR, et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nature Communications. 2019;10(1):4857. DOI: https://doi.org/10.1038/s41467-019-12536-4
34. Edwards TL, Giri A, Hellwege JN, et al. A trans-ethnic genome-wide association study of uterine fibroids. Frontiers in Genetics. 2019;10:511. DOI: https://doi.org/10.3389/fgene.2019.00511
35. Sakai K, Tanikawa C, Hirasawa A, et al. Identification of a novel uterine leiomyoma GWAS locus in a Japanese population. Scientific Reports. 2020;10(1):1197. DOI: https://doi.org/10.1038/s41598-020-58066-8
36. Sun K, Xie Y, Zhao N, et al. A case-control study of the relationship between visceral fat and development of uterine fibroids. Experimental and Therapeutic Medicine. 2019;18(1):404-10. DOI: https://doi.org/10.3892/etm.2019.7575
37. Ciavattini A, Di Giuseppe J, Stortoni P, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstetrics and Gynecology International. 2013;2013:173184. DOI: https://doi.org/10.1155/2013/173184
38. Qin H, Lin Z, Vasquez E, et al. Association between obesity and the risk of uterine fibroids: a systematic review and meta-analysis. Journal of Epidemiology and Community Health. 2021;75:197-204. DOI: https://doi.org/10.1136/jech-2019-213364
39. Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clinical Obstetrics and Gynecology. 2016;59(1):2-24. DOI: https://doi.org/10.1097/GRF.0000000000000164
40. Wong JY, Gold EB, Johnson WO, et al. Circulating sex hormones and risk of uterine fibroids: Study of Women's Health Across the Nation (SWAN). Journal of Clinical Endocrinology and Metabolism. 2016;101(1):123-30. DOI: https://doi.org/10.1210/jc.2015-2935
41. Yang Q, Mas A, Diamond MP, et al. The mechanism and function of epigenetics in uterine leiomyoma development. Reproductive Sciences. 2016;23(2):163-75. DOI: https://doi.org/10.1177/1933719115584449
42. Maekawa R, Sato S, Yamagata Y, et al. Genome-wide DNA methylation analysis reveals a potential mechanism for the pathogenesis and development of uterine leiomyomas. PLoS One. 2013;8(6):e66632. DOI: https://doi.org/10.1371/journal.pone.0066632
43. Alleyne AT, Bideau VS. Haplotypes of CYP1B1 and CCDC57 genes in an Afro-Caribbean female population with uterine leiomyoma. Molecular Biology Reports. 2019;46(3):3299-306. DOI: https://doi.org/10.1007/s11033-019-04790-y
44. Bideau VS, Alleyne AT. Leu/Val SNP polymorphism of CYP1B1 and risk of uterine leiomyoma in a Black population. Tumour Biology. 2016;37(3):4035-40. DOI: https://doi.org/10.1007/s13277-015-4239-8
45. Huang PC, Li WF, Liao PC, et al. Risk for estrogen-dependent diseases in relation to phthalate exposure and polymorphisms of CYP17A1 and estrogen receptor genes. Environmental Science and Pollution Research. 2014;21(24):13964-73. DOI: https://doi.org/10.1007/s11356-014-3260-6
46. Toprak M, Ates O, Ozsoy AZ, et al. Analysis of estrogen and progesterone receptor gene polymorphisms in leiomyoma. Journal of Clinical Laboratory Analysis. 2019;33(3):e22704. DOI: https://doi.org/10.1002/jcla.22704
47. Hsieh YY, Wang YK, Chang CC, et al. Estrogen receptor alpha-351 XbaI*G and -397 PvuII*C-related genotypes and alleles are associated with higher susceptibilities of endometriosis and leiomyoma. Molecular Human Reproduction. 2007;13(2):117-22. DOI: https://doi.org/10.1093/molehr/gal099
48. Denschlag D, Bentz EK, Hefler L, et al. Genotype distribution of estrogen receptor-alpha, catechol-O-methyltransferase, and cytochrome P450 17 gene polymorphisms in Caucasian women with uterine leiomyomas. Fertility and Sterility. 2006;85(2):462-7. DOI: https://doi.org/10.1016/j.fertnstert.2005.07.1308
49. Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-a polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertility and Sterility. 2006;86(3):686-93. DOI: https://doi.org/10.1016/j.fertnstert.2006.01.052
50. Villanova FE, Andrade PM, Otsuka AY, et al. Estrogen receptor alpha polymorphism and susceptibility to uterine leiomyoma. Steroids. 2006;71(11-12):960-5. DOI: https://doi.org/10.1016/j.steroids.2006.07.005
51. Veronica M, Ali A, Venkateshwari A, et al. Association of estrogen and progesterone receptor gene polymorphisms and their respective hormones in uterine leiomyomas. Tumor Biology. 2016;37(6):8067-74. DOI: https://doi.org/10.1007/s13277-015-4711-5
52. Zhai XD, Ye Y, Yang Y, et al. No association between estrogen receptor beta polymorphisms and uterine leiomyoma. DNA and Cell Biology. 2009;28(12):633-6. DOI: https://doi.org/10.1089/dna.2009.0917
53. Fischer C, Juhasz-Boess I, Lattrich C, et al. Estrogen receptor beta gene polymorphisms and susceptibility to uterine fibroids. Gynecological Endocrinology. 2010;26(1):4-9. DOI: https://doi.org/10.3109/09513590903159573
54. Kasap B, Turhan NO, Edgunlu T, et al. G-protein-coupled estrogen receptor-30 gene polymorphisms are associated with uterine leiomyoma risk. Bosnian Journal of Basic Medical Sciences. 2016;16(1):39-45. DOI: https://doi.org/10.17305/bjbms.2016.683
55. da Silva F, Pabalan N, Ekaratcharoenchai N, et al. PROGINS polymorphism of the progesterone receptor gene and the susceptibility to uterine leiomyomas: a systematic review and meta-analysis. Genetic Testing and Molecular Biomarkers. 2018;22(5):295-301. DOI: https://doi.org/10.1089/gtmb.2017.0233