Study of the cytokine genes SNPs association with the characteristics of the immunological status of children with recurrent respiratory infection
Aннотация
Background: Herpesvirus infections are one of the most common human infections that play a special role in children with recurrent respiratory infection cohort formation. The immunological status of such children is characterized by an imbalance in all links of immunity. The aim of the study: To study the immunological status of RRI children and to analyze the association of SNP -330T>G in IL2 and -1082G>A in IL10 with the relative and absolute values of the main subpopulations of lymphocytes. Materials and methods: In our study, we enrolled 76 children between 10 and 17 years old who had acute respiratory infections at least 5 times a year. Flow cytometry was used for lymphocyte subpopulations analysis. Polymorphic variants were determined by allele-specific PCR. Results: In anti-NA EBV IgG and anti-CMV IgG positive children with AA genotype for the -1082G>A IL10 polymorphism the average relative number of B-lymphocytes was 9.6%, which is below normal values (р=0.01). The average number of B-lymphocytes for heterozygotes was 12.4% (р=0.01). In the subgroup children with GG genotype, the average number of B-lymphocytes was 16.7% (р=0.01). In children with AA genotype the average level of NK cells was 19.3% (р=0.04). Children with GG genotype had lower level of NK cells (10.9%) (р=0.04). Conclusion: Our studies revealed a relationship between the immune status indicators and the genotypes for the -1082G>A IL10 polymorphism in anti-NA EBV IgG and anti-CMV IgG positive RRI children group. The -1082AA genotype of the IL10 is associated with a decrease in the relative and absolute number of B-lymphocytes, as well as with an increase in the relative number of NK cells. RRI children with -1082GG genotype tend to increase the relative and absolute number of B-lymphocytes, and to decrease the relative number of NK-cells
К сожалению, текст статьи доступен только на Английском
Introduction. Herpesvirus infections are one of the most common human infections that play a special role in children with recurrent respiratory infection cohort formation. Children with recurrent respiratory infection (RRI) have a higher incidence of acute respiratory disease (ARI) than the general population of children of the same age group. According to some authors, the category of RRI children includes those who have acute respiratory infections more than 5 times during the year; according to other authors – 8 or more times [1, 2, 3]. The practice of classifying children as RRI depending on age and disease relapses number has become widespread. According to this practice the presence of eight or more relapses of infections per year in children under three years of age, or six or more cases in children over three years of age allows to classify children as with RRI [4].
The prevalence of Epstein-Barr viral infection and cytomegalovirus infection both in the adult population and in children remains high not only in Russia, but also worldwide. Specific immunoglobulins for the Epstein-Barr virus and cytomegalovirus are detected in the vast majority of the adult population, in some regions this indicator reaches 90% and higher [5, 6, 7]. The percentage of children infected with the Epstein-Barr virus and cytomegalovirus depends on age, but on average remains quite high, about 70% for the Epstein-Barr virus infection [7, 8].
Herpesvirus infections are capable to diminish the antiviral immune response of the body, namely, they are able to evade T-cell recognition or stimulate the mutual destruction of T-cytotoxic lymphocytes, induce a decrease in their number, including by apoptosis; inhibit the action of the complement system, dendritic cells, antibodies; disrupt the transcription of interferon-stimulated genes and the presentation of viral antigens by HLA-I molecules; cause an imbalance of both cellular and humoral immunity [11-16]. One of the mechanisms contributing to the human body immune response inhibition to the herpes virus penetration is a host cytokine profile change due to the viral expression of cytokine-like proteins, such as IL-10 or IL-6 (EBV, CMV) [6, 17, 18].
Cytokines regulate intercellular interactions between human immune system cells during the immune response to virus penetration, including herpes viruses [19]. Allelic variants of cytokine genes determine the level of these molecules in human blood, which can affect the body's immune response, the clinical course and outcome of the disease, as well as the body's resistance to various viruses, including herpes viruses [20, 21].
IL10 is an immunosuppressive anti-inflammatory cytokine produced by activated T lymphocytes, including Th2, B lymphocytes, and monocyte / macrophage cells [17]. IL10 suppresses the macrophages, monocytes, and dendritic cell`s function, reducing their production of pro-inflammatory cytokines, decreases the expression of HLA-II genes, and indirectly suppresses the activity of T-lymphocytes. Although IL10 stimulates the proliferation and differentiation of B-lymphocytes, its overall effect is usually immunosuppressive [6, 22, 23, 24].
At the same time, the effect of IL-10 in the antiviral immune response can be more complex and depend on such factors as the place of virus penetration and the infectious process stage. For example, high plasma levels of IL-10 may play a protective role in early responses to human immunodeficiency virus, but persistent expression may contribute to chronic infections. In persistent infection, IL10 expression affects the antigen-presenting ability of dendritic cells and causes T cells depletion and inhibition of their functions [17].
Promoter region of the IL10 gene has several single nucleotide substitutions (-592A>C, -819T>C, -1082G>A), which are associated with increased expression of this cytokine. Homozygotes for the G allele of the -1082G>A polymorphism have an increased IL10 expression level, heterozygotes have an average expression level, and homozygotes for the A allele demonstrate a low expression level [22, 25].
IL2 or T cell growth factor refers to cytokines produced predominantly by Th1, as well as by T cytotoxic, NK cells and dendritic cells. IL2 stimulates the proliferation and differentiation of B and T cells, NK lymphocytes, and can promote induced cell death [26]. There is one SNP (-330T>G) in the promoter region of the IL2 gene. The T allele and the TT genotype are associated with increased IL2 production and a possible protective effect against herpesvirus infections [27, 28].
Thus, IL2 and IL10 regulate the intensity and duration of the immune response, form the characteristics of the immune response and the susceptibility to viral infections. SNP -330T>G in the IL2 gene and -1082G>A in the IL10 gene affect the levels of genes transcription [29,30,31]. There are no studies concerning the analysis of the association of IL2 and IL10 genes genotypes with changes in lymphocyte subpopulations in CMV and EBV mix infections. In this regard, the aim of this work was to study the immunological status of RRI children and to analyze the association of SNP -330T>G in the IL2 gene and -1082G>A in the IL10 gene with the relative and absolute values of the main subpopulations of lymphocytes (CD3, CD4, CD8, CD16, CD19).
Materials and methods. Samples for this study were collected at the Child Health Center No. 4 and Diagnostic Laboratory «Nauka», Rostov-on-Don (Russia). A total of 76 children, 56% boys and 44% girls, between 10 and 17 years old who had acute respiratory infections at least 5 times a year were enrolled in the study. Children with chronic respiratory diseases or children who demonstrated immunodeficiency were ruled out.
All the patients (for patients under 15 years old, the consent of legal representatives was obtained) gave their informed consent to the participation in the study, which was approved by the local Ethical Committee. All procedures were in accordance with the Declaration of Helsinki as revised in 2013.
Whole peripheral blood stabilized with K2-EDTA was used for immunological and molecular genetic studies. Blood serum was obtained for enzyme-linked immunosorbent assay.
IgG to cytomegalovirus and IgG to the nuclear antigen NA of the Epstein-Barr virus were analyzed by enzyme-linked immunosorbent assay with VektorCMV-IgG and VectorVEB-NA-IgG reagent kits (Vector-Best, Russia). Measurement of optical density in the wells was performed on a semi-automatic ELISA analyzer Infinite F50 (Tecan, Austria) at a wavelength of 450 nm.
Flow cytometry was used for lymphocyte subpopulations (B-lymphocytes, T-lymphocytes (T-helpers, T-cytotoxic), and NK cells) analysis. The analysis was performed on the Navios flow cytometer (Beckman Coulter, USA) using a mixture of tetraCHROME ™ CD45 / CD4 / CD8 / CD3 antibodies (Beckman Coulter, USA) and a mixture of tetraCHROME ™ CD45 / CD56 / CD19 / CD3 antibodies (Beckman Coulter, USA).
Amplification products were separated in 2% agarose gel by horizontal electrophoresis. The results were visualized using a Gel Doc XR + UV transilluminator (Bio-Rad, USA).
Allele and genotype frequencies for the studied polymorphisms were calculated using free software (http://www.husdyr.kvl.dk/htm/kc/popgen/genetik/applets/kitest.htm). Hardy-Weinberg equilibrium (HWE) and comparisons among groups were evaluated by the Chi-square (χ2) test with a 95% confidence interval.
Quantitative variables were expressed as median and interquartile range or as percentage. The interquartile range is indicated as 25% and 75% percentiles. Comparisons of quantitative variables were made using the appropriate non-parametric Mann – Whitney test.
To conduct a correlation analysis between genotypes and indicators of the immunological status of RRI children, the Spearman correlation coefficient was used. Differences were considered statistically significant at a significance level of p <0.05.
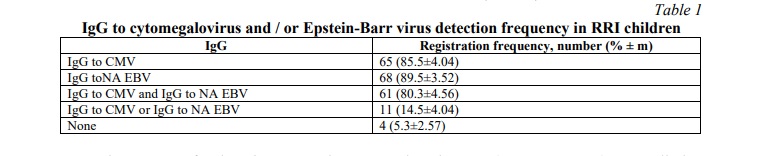
Results. Of the 76 RRI children the CMV IgG test was positive in 65 (85.5% cases). IgG to the NA EBV nuclear antigen were detected in 68 (89.54%) cases. At the same time, 61 children (80.3%) had both IgG to CMV and IgG to the nuclear antigen NA EBV, and 4 children (5.3%) did not have both tested IgGs (Table 1).
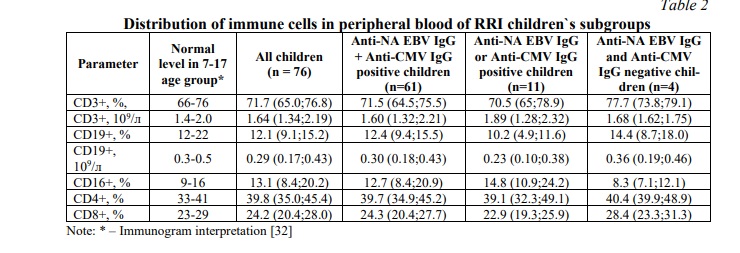
The content of B-lymphocytes, T-lymphocytes, NK-cells in RRI children corresponds to the normative parameters of the immunogram (Table 2). Immunological study did not reveal significant differences in the relative and absolute number of B-lymphocytes, T-lymphocytes (CD4 +, CD8 +), NK-cells between groups of RRI children (Table 2).
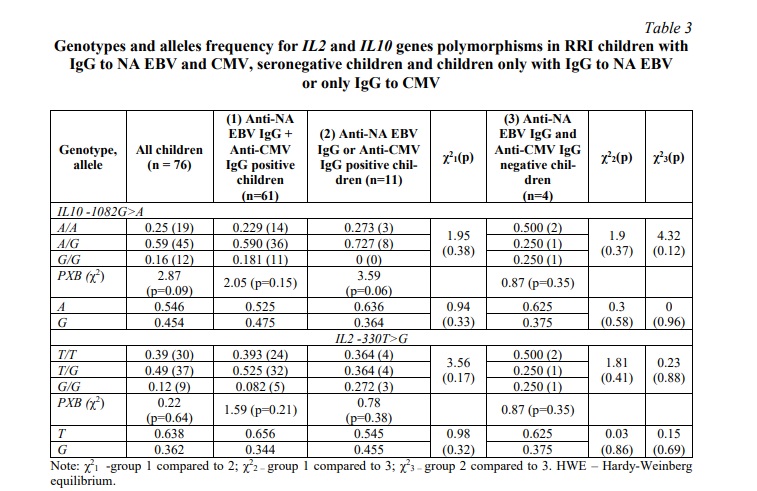
The genotype distribution of the IL2 and IL10 gene polymorphisms were in accordance with the Hardy-Weinberg equilibrium in all studied groups of children (Table 3).
Relative and absolute number of B-lymphocytes in the general group of subjects and among RRI anti-NA EBV IgG and anti-CMV IgG positive children was associated with -1082G>AIL10 genotype (Table 4). In anti-NA EBV IgG and anti-CMV IgG positive children with AA genotype the average relative number of B-lymphocytes was 9.6%, which is below normal values (Fig. 1). Only 35.7% blood samples of anti-NA EBV IgG and anti-CMV IgG positive children with AA genotype corresponded to the reference values of B-lymphocytes number. At the same time, in heterozygotes for the -1082G>AIL10 gene polymorphism, 52.8% of anti-NA EBV IgG and anti-CMV IgG positive children were characterized by normal B-cell levels. The average number of B-lymphocytes for heterozygotes was 12.4% (Fig. 1). In the subgroup anti-NA EBV IgG and anti-CMV IgG positive children with GG genotype, the average number of B-lymphocytes was 16.7%. Only in this subgroup were children with B-cell levels exceeding the reference values (Fig. 1). Thus, it was found that in anti-NA EBV IgG and anti-CMV IgG positive children, the level of B-lymphocytes correlates with the genotype for the -1082G>A polymorphism of the IL10 gene.


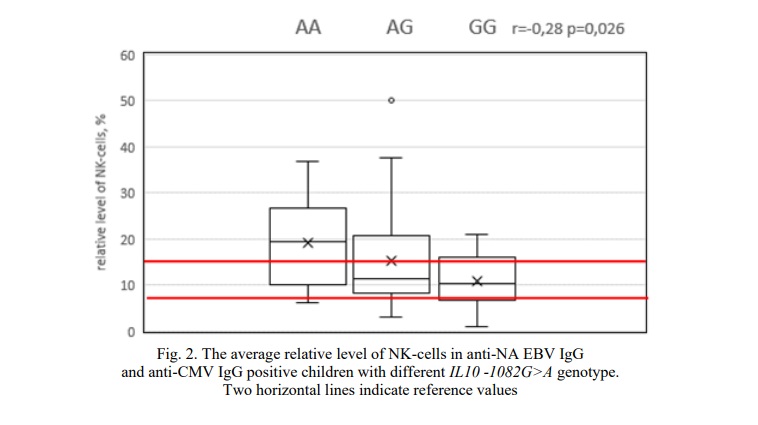
In addition, in the group of anti-NA EBV IgG and anti-CMV IgG positive RRI children an inverse relationship was found between -1082G>A IL10 polymorphism and the relative number of NK cells (p <0.05) (Fig. 2). In children with AA genotype the average level of NK cells was 19.3% (Fig. 2). In 57.2% of cases the relative number of NK cells was higher than the reference values, in 21.4% cases children with the AA genotype had normal and reduced values of NK cells. In heterozygotes the average level of NK cells was 15.3%. Children with GG genotype had even lower average level of NK cells (10.9%) (Fig. 2). At the same time, only in 18.1% of cases the relative number of NK cells was above reference values (Fig. 2).
The highest correlation coefficients were found for a subgroup of Anti-NA EBV IgG and Anti-CMV IgG negative children. However, the small size of this subgroup does not allow us to speak about the significance of this correlation. Still, there are several facts worth paying attention to. First, the age of children in this subgroup ranged from 11 to 17 years. Secondly, three out of 4 children are homozygous for both studied SNPs of cytokine genes (1 child has the -330AA IL2 / -1082GG IL10 genotype; 2 children have the -330TТ IL2 / -1082АA IL10 genotype). Further research is required to investigate the possible relationship between the immunological status of RRI seronegative children and their genotype for allelic variants of cytokines genes and other immune system factors.
Discussion. Persistent viruses, including herpes viruses, affect all components of the body's immune defense. Herpes viruses disrupt the immune cells balance, providing an opportunity not only to penetrate the human body, thereby causing an acute infection, but also have passive and active self-defense systems that cause long-term latent or chronic infections. Persistence of herpes viruses is often characterized by immune cells imbalance and their functional insufficiency [33]. It is known that viral IL-10 synthesized by EBV and CMV exhibits inhibitory activity identical to human IL-10 [17, 34, 35]. However, viral IL-10 lacks immunostimulating properties [36, 37].
Viruses are eliminated from the human body through the mechanism of NK cell activation [38]. Latent cytomegalovirus and Epstein-Barr viral infections are associated with a reduced level of NK cell production, which is one of the factors in establishing viral persistence [39,40,41]. In addition, the Epstein-Barr viral infection is often characterized by suppression of the T-cell link of immunity, T-lymphocytes and T-helpers number decrease, T-cytotoxic cells increase, as well as an IRI index decrease [40]. The data on the immunological status of cytomegalovirus infected children are contradictory, but there is animbalance in the cellular component of immunity. Cytomegalovirus infected RRI children significantly differ incellular immunity indicators fromRRI children without confirmed cytomegalovirus disease [39].
In the framework of our study, the levels of T cells, B lymphocytes, and NK cells detected were not specific for Anti-NA EBV IgG or Anti-CMV IgG positive RRI children, but observed in RRI children, regardless of their serological status. We have not revealed any strictly specific changes caused by the transferred cytomegalovirus and Epstein-Barr viral infections.
The relationships between the genetic component immune system and the immune status of RRI children in connection with CMV or EBV infection seem to be insufficiently studied. There are very few works devoted to this topic, most of them are aimed at studying certain aspects of the immune status of children with CMV or EBV, without studying the genetic component [29, 31]. There are no studies concerning the analysis of the association of IL2 and IL10 genes genotypes with changes in lymphocyte subpopulations in CMV and EBV mix infections.
Our studies revealed a certain relationship between the immune status indicators and the genotypes for the -1082G>A IL10 gene polymorphism in anti-NA EBV IgG and anti-CMV IgG positive RRI children group.
IL10 is a key anti-inflammatory cytokine produced by activated immune cells. The effects of IL10 on different immune cells are varied. It inhibits the expression of proinflammatory cytokines by T-helper type 1, NK-cells, monocytes, macrophages and dendritic cells and inhibits the activation of T-helpers. At the same time, IL10 activates the proliferation and differentiation of T-cytotoxic, B-lymphocytes and antibodies production [6, 22, 23, 24, 42, 43, 44]. The level of interleukin 10 content, in turn, can influence the body's resistance to infection by a number of viruses, including herpes viruses, as well as the possibility of developing latent infection, its chronicity and reactivation [22, 23, 43].
Polymorphism -1082G>A (rs 1800896) IL10 affects the gene expression level. Thus, the GG genotype is associated with increased cytokine production, a more distinct dysregulationin the humoral link of immunity and a decrease in the antiviral defense of the body as a whole [6, 23, 24, 44].
Increased IL10 production can help prevent infection. The explanation for persistent seronegativeness in some subjects is the destruction of the virus at the onset of infection [17, 22, 45, 46]. With a later course of infection, increased secretion of IL10, in turn, is one of the factors in the development of persistence and disease chronicity, since an IL10 excess leads to a decrease in the body's anti-infective defense [23, 43].
IL10 regulates the survival, proliferation and differentiation of B cells (by stimulating the expression of hTERT, activating telomerase in B cells, stimulating the differentiation of plasma cells due to memory B cells) [17, 35, 47, 48]. Thus, it can be assumed that the increased production of IL10 in the -1082GG genotype may contribute to an increase in the total number of B-lymphocytes.
According to the literature, it is known that IL10 promotes the proliferation of NK cells and can act as a survival factor for activated NK cells [17, 49]. At the same time, some authors have noted the ability of IL10 to suppress the activation of NK cells and their production of proinflammatory cytokines, including IFNγ [50, 51].
It can be assumed that cytokine-like proteins of viral origin, or rather the ratio of concentrations and activity of human and viral molecules, play a certain role in the spectrum formation and level of immune cells in RRI children. The correlations we revealed between the SNP genotype of the IL10, and the number of B lymphocytes and NK cells are characteristic for the subgroup of children with IgG to CMV and NA EBV. These viruses can produce cytokine-like compounds that affect human immune system functioning. Researchers note a short-term effect of CMV IL-10 on human NK cells leading to a significant increase in their cytotoxicity [52]. CMV IL-10 and EBV IL-10 stimulate the survival and proliferation of B-lymphocytes [17, 53].
A relative change in the subpopulation composition of lymphocytes, namely, a decrease in the number of NK cells and a simultaneous increase in the number of B lymphocytes, can probably contribute to the chronicity of the inflammatory process. An imbalance in the production of cytokines between Th1 and Th2 towards the predominance of Th2 production, an increase in the population of B cells, and the production of antibodies contributes to a shift towards the humoral link of immunity, as well as viral persistence and the transition of the disease to the chronic stage [6]. In addition, the lack of NK cells, as one of the most important components of the innate defense of the body, designed to control the penetration of viruses, their destruction and elimination from the body leads to a decrease in the antiviral defense of the body at the early stages of the development of infection [17, 51, 54, 55].
Conclusion. The results of the present study found no significant difference between the -1082G>A IL10 gene polymorphism and the -330T>G IL2 gene polymorphism allele and genotype frequencies distribution for the studied groups of children. However, a correlation has been established between the immune status indicators of Anti-NA EBV IgG and Anti-CMV IgG positive RRI children, namely, the number of B cells and NK cells, and genotypes for the -1082G>A (rs 1800896) IL10 polymorphism. The revealed correlations require verification, confirmation on large samples. It is also necessary to analyze the presence of such correlations with the presence of antibodies to other infectious agents.
Financial support
This study was funded by the Ministry of Science and Higher Education of the Russian Federation № 0852-2020-0028 and was performed with the equipment of the Center of collective use «High technologies» (Southern Federal University).





















Список литературы
Список использованной литературы появится позже.