Comorbidity and syntropy of benign proliferative diseases of the female reproductive system: non-genetic, genetic, and epigenetic factors (review)
Aннотация
Background: Benign proliferative diseases of the female reproductive system (endometriosis, endometrial hyperplasia, and uterine leiomyoma) are comorbid and often occur together. The aim of the study: To establish common risk factors for the development of benign proliferative diseases of the female reproductive system (endometriosis, endometrial hyperplasia, and uterine leiomyoma). Materials and methods: The review includes up-to-date data from articles found in PubMed and Elibrary on this topic. Results: The article reviews the recent literature about factors determining comorbidity and syntropy of the diseases. Their comorbidity may be based on common environmental risk factors (early menarche, body mass index, induced abortions, etc.) and mechanisms of the pathogenesis, which include hormone-dependent cellular proliferation associated with the action of sex hormones and hormone-independent cellular proliferation processes stimulated by growth factors and cytokines, apoptosis, and pathological neoangiogenesis, etc. These common mechanisms are backed by syntropic genes (e.g., FSHB, COMT). Syntropy and pleiotropy appear to be closely related: syntropic genes manifest pleiotropic effects too. Also, epigenetic factors, such as miRNAs are important determinants of the diseased phenotypes. Conclusion: Identification of the shared factors contributing to the benign proliferative diseases of the female reproductive system helps to determine targets for therapeutic intervention and efficient control of the comorbidity.
К сожалению, текст статьи доступен только на Английском
Introduction. Benign proliferative diseases of the female reproductive system – endometriosis, endometrial hyperplasia, and uterine leiomyoma, prevail in the structure of the overall gynecological morbidity [1, 2, 3]. Uterine leiomyoma (UL), commonly known as fibroids, is a benign tumor of the smooth muscle in the uterus [1, 4]. About 30 percent of reproductive-age women are diagnosed with UL, which is considered as a major factor of gynecologic morbidity, one of the primary causes of hospitalizations for gynecological disorders, and the most frequent reason for hysterectomy in the USA [1]. Endometriosis (EM) is a condition when endometrial tissue proliferates in ectopic locations, such as the pelvic peritoneum, ovaries, and rectovaginal septum [3, 5]. Endometriosis is diagnosed in 6-10% of women of reproductive age and in 20-50% of infertile women [5, 6]. Endometrial hyperplasia (EH) is defined as excessive growth and thickening of the endometrium. This process increases the glands/stroma ratio [7, 8]. In the structure of gynecological morbidity in women of reproductive age, EH possesses a significant segment. In developed countries, there are an estimated 200,000 new cases of EH per annum [2].
Benign proliferative diseases of the female reproductive system are comorbid and quite commonly occur together [9-12]. Nezhat et al. [9] reported the co-occurrence of uterine leiomyoma and endometriosis in 87.1% of women who underwent laparoscopic myomectomy/hysterectomy for symptomatic uterine leiomyoma. Simple endometrial hyperplasia occurs in 22.7% of patients with uterine leiomyoma [10]. Among patients with endometrial hyperplasia, uterine fibroid occurs in 51.54% and endometriosis in 35.19% [11], with uterine fibroid occurring in 52.40% and endometrial hyperplasia in 46.33% of patients with endometriosis [12].
The aim of the study. To establish common risk factors for the development of benign proliferative diseases of the female reproductive system (endometriosis, endometrial hyperplasia, and uterine leiomyoma).
Materials and methods: The review includes up-to-date data from articles found in PubMed and Elibrary on this topic.
Results and Discussion. One of the causes for comorbidity of the above benign proliferative diseases may be common environmental and/or endogenous risk factors. Risk factors for UL are endogenous hormones, early menarche or late menopause, obesity, induced abortions, family history, genetic factors, polycystic ovary syndrome, age, ethnicity, oral contraceptives, lifestyle, diet, and others (Fig. 1, 2, 3) [1, 4, 13, 14, 15].
The continuous overstimulation of the endometrium by estrogen was recognized among the primary factors of the EH pathophysiology [8]. The other EH risk factors are quite diverse and include early menarche or late menopause, overweight, diabetes mellitus, infertility, hypertension, family history, genetic factors, induced abortions, chronic anovulation, polycystic ovary syndrome, tamoxifen therapy, estrogen hormone replacement therapy, postmenopausal status, and others [2, 7, 8, 11].
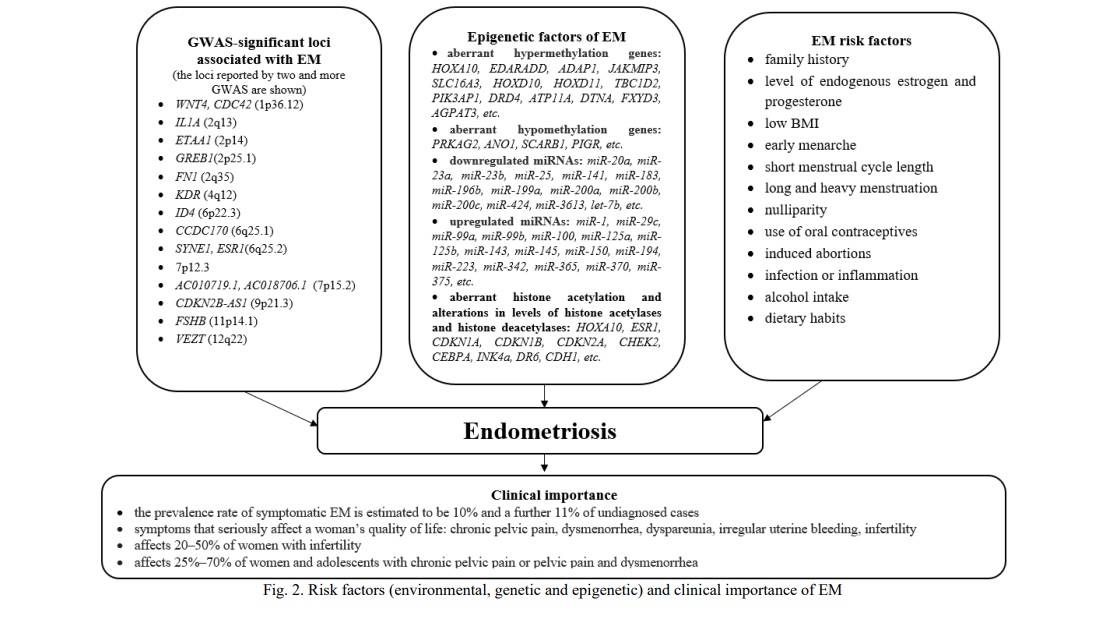
Endometriosis was associated with body weight, age at menarche, duration of menstrual discharge and menstrual cycle, reproductive factors, family history, genetic factors, induced abortions, diet, and others [3, 5, 6, 12, 16, 17, 18].
In general, it appears that factors affecting the level of endogenous estrogen (early menarche, body mass index, and others), family history, genetic factors, induced abortions, etc., are shared factors for the risk of benign proliferative disorders of the female reproductive system (uterine leiomyoma, endometriosis, and endometrial hyperplasia) and can determine their comorbidity.
At the same time, benign proliferative diseases of the female reproductive system, along with common risk factors, have common pathogenetic mechanisms of development: hormone-dependent cellular proliferation associated with the action of sex hormones (absolute, relative and local hyperestrogenism); hormone-independent cellular proliferation processes, stimulated by growth factors and cytokines, and causing uncontrolled cellular proliferation, apoptosis, and pathological neoangiogenesis; processes involving pro- and anti-inflammatory cytokines caused by genital infections, invasive intrauterine interventions; immune disorders associated with immunological resistance, etc. [1-4, 7, 8].
Age at menarche has been suggested as a risk factor for UL, EH, and EM [1, 2, 7, 18]. Women with early onset of menarche have, on average, a longer period of menstruations in their life and thus a greater overall exposure to estrogens, which may induce the growth of UL and promote EH [1, 7]. Cell division rate in the myometrium is highest during the luteal phase of the menstrual cycle, therefore, a longer history of cycling may increase the risk of UL [1]. Continuing exposure to estrogens in the absence of progesterone seems to promote the development of EH [2]. Likewise, an increased lifelong exposure to menstruation due to early menarche may also increase the risk of EM [18]. The relationship between age at menarche and the proliferative benign diseases is further supported by that candidate genes for age at menarche were associated with EH and EM [11, 12, 19].
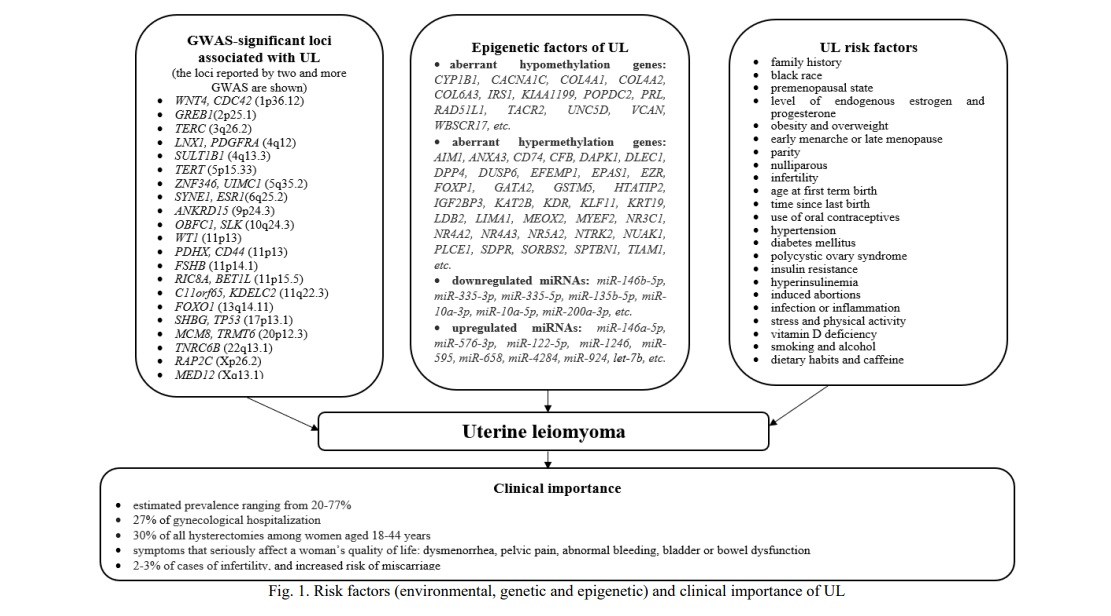

Age at menopause has also been suggested to affect the risk of UL and EH [1, 2, 7]. Late menopause has a similar effect to early menarche: it extends a lifelong exposure to estrogens, which may promote the growth of UL and promote EH [1, 7].
One more risk factor for UL and EH is obesity/overweight/high body mass index [1, 2, 7, 8, 14, 15]. A direct correlation between BMI and UL was reported: the risk increased by 20% per each 10 kg of weight gain [14]. Obese women (BMI >30 kg/m2) showed a nearly 4-fold increase in the incidence of atypical EH, as compared to non-obese ones. Furthermore, women with BMI of 40 kg/m2 had a 13-fold higher risk of atypical EH and a 23-fold increased risk of non-atypical EH as compared to the women with normal BMI [8]. This relationship can be explained by that androgens produced by the adrenal glands and ovaries are converted into estrogens in the fat tissue. Excessive fat promotes the development of insulin resistance and reduced synthesis of globulin, which binds sex hormones [8]. As a result, obese women have a higher level of free circulating estrogens that can promote the development of UL and EH. Besides, hyperinsulinemia, one of the main elements of the metabolic syndrome, is associated with higher serum levels of the insulin-like growth factor-1 and epidermal growth factor [20], which, in turn, induce secretion of estrogen in ovaries or promote cell proliferation in the endometrium and myometrium [1, 2].
BMI is a risk factor for EM too, but the effect is opposite to the mentioned above: low BMI increases the risk of EM [5, 17]. The recent meta-analysis reported that the EM risk decreased (OR=0.67) per each 5 kg/m2 BMI gain [17]. One of the possible explanations of these results is that obese women usually have a higher estrogen level that may lead to anovulatory and irregular menses and, respectively, lower chances of the retrograde menstruation [16].
The history of induced abortions significantly increases the risk of UL, EH, and EM [11, 12, 13, 16]. Induced abortions in the first trimester of pregnancy may cause multiple health complications, including polycystic ovary syndrome, hyperprolactinemia, anovulatory menstruation, post-abortion endometritis, and the others [21]. Induced recurring abortions result in injuries of the uterus. Uterine injuries may induce the expression of the growth factor that speed-ups cellular proliferation, suppresses apoptosis, and increases production of the extracellular matrix [1]. The risk for UL, EH, and EM directly correlates with the number of abortions in the anamnesis [11, 12, 13]. A higher number of induced abortions was associated with an increased UL risk (one induced abortion - OR = 1.32, two induced abortions - OR = 1.45, and ≥3 induced abortions - OR = 1.62) [13], EH (it increases from OR=2.00 in the case of two abortions up to OR=6.54 with four and more abortions) [11] and EM (it increases from OR = 2.05 in the case of 2 abortions up to OR = 5.87 with 4 and more abortions) [12]. Induced abortions may also be associated with other hormone-related diseases (e.g., breast cancer) [22].
Another important risk factor for UL, EH, and EM is a family history of the disease [1-4]. A risk to develop UL is 2.5-fold greater for first-degree relatives of affected women than the population average [23] and the concordance among monozygotic twins is almost twice that of dizygotic twins [15]. Heritability estimates of UL from twin studies vary from 26 to 69% in European populations [15]. Genetic factors are important for the development of EM [3, 6]. Results of twin studies estimate their contribution at 51% [6]. As to the relative contribution of genetic factors to the development of EH, no family or twin studies have been conducted so far and candidate gene association studies were limited [7, 11].
Recent genome-wide studies (GWAS) provided more estimates for genetic factors of the diseases. The current National Genome Research Institute's catalog of GWAS (https://www.ebi.ac.uk/gwas/) includes more than 10 studies of EM [e.g., 24-31]. These studies reported more than 20 significant loci associated with EM. Among those, 19 SNPs were estimated to determine 5.19% of the EM variability [30].
Several GWAS of UL were conducted in different ethnic populations, including Japanese, Caucasians of the USA and Australia, and African-American women [e.g., 31-36]. In 2018, two teams presented results of the first GWAS of UL in European populations [33, 34]. Both studies reported somewhat similar results: 21 and 22 GWAS-significant loci for UL, respectively. The contribution of these 21 GWAS-significant SNPs to the UL variability was estimated at 13% [33]. A follow-up GWAS meta-analysis of 35,474 cases and 267,505 controls of European ancestry confirmed 21 previously reported loci and identified eight novel loci for UL [35]. Besides, four loci identified in the meta-analysis were also associated with EM risk: 1p36.12 (WNT4/ CDC42), 2p25.1 (GREB1), 6q25.2 (ESR1), 11p14.1 (FSHB). A summary of the candidate genes for UL and EM determined by GWAS is given in Table 1

An epidemiological meta-analysis of 402,868 women suggested at least a two-fold higher risk for UL in patients with a history of EM [35]. It was hypothesized that both UL and EM were underlain by hormone-related factors [33] and thus might have a shared genetic basis [35]. This assumption was supported by the recent findings of syntropic genes for these diseases [33, 35]. In particular, the GWAS of UL in European populations [33] replicated three (GREB1, CDC42/WNT4, SYNE1/ESR1) of the 19 previously reported candidate loci for EM [30].
A comparison of the biological processes for the EM and UL development (gene expression, gene networks, and epigenetic regulation) revealed their similarities thus suggesting molecular syntropy of both disorders. The PANTHER overrepresentation test of the biological pathways for the syntropic genes provided further support for this assumption (Table 2).
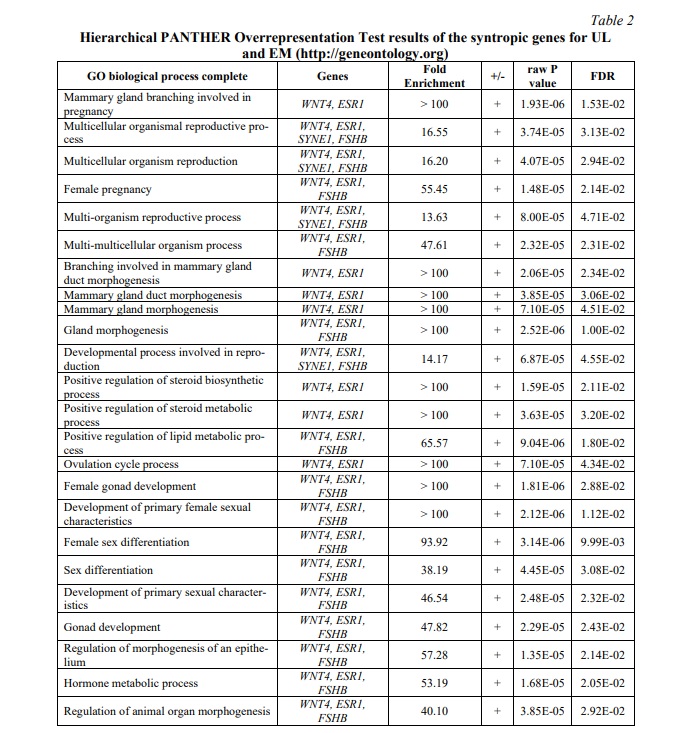
No GWAS on EH has been conducted so far. Candidate gene studies reported associations of tumor necrosis factors and their receptors (TNFα, Ltα, TNFR1, TNFR2), chemokines (MIP1β, MCP1, SDF1), growth factors (ТGFb1, IGF1, VEGF, FGFR2), interleukins (IL-6, IL-10, IL-5), folate metabolizing and detoxifying enzymes (TYMS, ApoE, GSTPI, CYPIAI, СYP1А2,SULT1А1, GP-IIIa), candidate genes for age at menarche, and others [7, 11]. Some of these genes were also associated with EM and UL [3, 37, 38].
The available body of evidence suggests that FSHB can be one of the syntropic genes for UL, EH, and EM. The meta-analysis of 11 GWAS reported the region on chromosome 11 (11p14.1, rs74485684) harboring the FSHB gene as that associated with EM [30]. This variant was replicated by GWAS of UL in European populations [33]. Several loci associated with EM, including rs11031006 located in 11p14.1 harboring the FSHB gene, were reported as candidates for UL in the recent GWAS [35]. Polymorphisms of the FSHB gene (rs555621, rs11031010, rs1782507) were also documented for their association with EH [11, 12]. Importantly, rs11031010 FSHB associated with EH is located just 1.9 kb from rs74485684 (associated with EM) and linked to rs11031006 (r2 = 0.64) (associated with EM [30] and UL [35] respectively).
There is evidence that some tightly linked polymorphisms of the FSHB gene may contribute to various reproductive characters, including the serum level of the follicle-stimulating and luteinizing hormones [39], ages at menarche [40] and menopause [41], BMI of adults [19]. Several SNPs in the 5’-region of FSHB were suggested to play a key role in various reproductive processes [42]. Importantly, three alleles of the FSHB gene (G of rs555621, А of rs11031010, and А of rs1782507) were associated with later menarche [40] and were suggested as a protective factor for EH [11]. That means the age at menarche and the EH risk may have a shared genetic basis.
The FSHB gene encodes a beta-subunit of the follicle-stimulating hormone. This hormone has multiple functions in the female reproductive system, including stimulation of the follicular granulosa cells proliferation, the rescue of follicles from apoptosis, synthesis of the luteinizing hormone receptors on these cells before ovulation, control of the synthesis of aromatases that convert androgens into estrogens (estradiol), etc. (http://www.ensembl.org/).
It seems quite reasonable to hypothesize that, in addition to FSHB and other candidate genes for the age at menarche, a syntropic effect for UL, EH, and EM may be manifested by genes contributing to those anthropometric traits, which were shown to increase risks for these diseases (e.g., height, BMI, and the others). For example, an increased BMI was reported to elevate a risk for EH [2, 7, 8] and UL [1, 14, 15], while a lower BMI might increase a risk for EM [5, 17]. Several genetic variants within or near genes CAB39L, GRB14, KIFAP3, and WNT4 were associated with both EM and the ratio of waist and hip circumference [43]. Several genes (e.g., FANCL, FTO, GPRC5B,LIN28B, MAP2K5, TNNI3K, and the others) were suggested to contribute to some of the above-mentioned phenotypes, such as the age at menarche, height, BMI, and the others [44, etc]. Some of these genes (e.g., LIN28B) were also associated with EH and EM [11, 12, 19].
Another syntropic candidate gene contributing to both anthropometric traits (age at menarche, BMI, and height) and the above-mentioned diseases (UL, EM, and EH) can be COMT. COMT (catechol-O-methyltransferase) is an enzyme involved in the estrogen metabolism. The rs4633 polymorphism of these gene was associated with AAM, height, and BMI [19]. This polymorphism and related rs4680 were associated with the expression level of COMT in peripheral blood [19]. The activity of the COMT enzyme is elevated in the human leiomyoma tissue as compared with the normal myometrium; since this enzyme plays a key role in estrogen metabolism, the observed association suggests a possible causal role of COMT in the UL formation [4]. In addition, the rs4680 polymorphism encodes a Val158Met substitution in COMT: the Met variant has a 40% lower activity as compared to Val [45]. Further support of the possible contribution of rs4680 to UL comes from the meta-analysis by Feng et al. [46], which reported its association with UL. This locus was also associated with EM [47], while rs4633 COMT – with EH [11].
The above results suggest that pleiotropic loci apparently account for a major contribution to the analyzed phenotypes (age at menarche, anthropometric characteristics, UL, EH, EM). In turn, this ample evidence suggests a shared genetic basis for these phenotypes. The estimates of genetic correlations (rg) between various phenotypes and EM based on the GWAS results provide a support for this assumption [30]. Indeed, the significant genetic correlations (p<0.05) were determined between EM and the age at menarche, height, and obesity. Furthermore, significant genetic correlations were obtained between the age at menarche and two anthropometric characteristics, BMI (rg= −0.35, p=1.4×10−91) and height (rg =0.13, p=2.5×10−10) [48]. The analysis of the data of 250,037 women from the UK Biobank yielded a strong association between the age at menarche and obesity (p<1×10−21), UL (p<1×10−21), and EM (p<7.48×10−5) [49]. A significant genetic correlation was determined between UL and EM (p = 0.003) by Rafnar et al. [33]. The existence of syntropic genes and probable shared genetic basis can be important factors of UL, EH, and EM comorbidity.
Another reason for the comorbidity of benign proliferative diseases of the female reproductive system (UL, EH, and EM) may be the involvement of common regulatory non-coding RNAs and, in particular, micro-RNAs (miRNAs) in their development [50]. MiRNAs modulate various biological processes occurring in the endo- and myometrium, such as cell proliferation, apoptosis, cell differentiation, and inflammatory response. These RNAs are involved in the regulation of the effect of steroid hormones on the endo- and myometrium. In the normal endometrium, the highest expression levels were reported for miR-21, miR-125b, miR-145, miR-23b, miR-26а, miR-29а, and miR-99а [50]. Thirty-two miRNAs were found to be differentially expressed in glandular epithelial and endometrial stromal cells. Steroid hormones had the strongest effect on the expression of miR-20а, miR-21 и miR-26а. In the endometrium, the elevated expression was observed for miR-29c and miR-145, and lower expression – for miR-196b, miR-199а, miR-23a/b. The differential expression of miRNAs resulted in the altered expression of the respective targeted proteins playing an important role in the pathogenesis of EM. For example, the elevated expression of miR-29c and miR-145 lowered the expression of COL7A1, UPK1B, TFAP2C и FASCIN1, JAM-A, PAI-1, while the lower expression of miR-196b и miR-199а increased transcription of BCL2, C-MYC, HOXA10, IKK/NFKB, and IL-8 [50].
The comparative analysis of the expression level of micro-RNA in the normal myometrium and cells of myomatous nodes demonstrated that, in the normal myometrium, the expression of miR-141, miR-200а, miR-200b, miR-200c, and miR-429 was elevated that determined the lower production of targeted proteins ZEB1, ZEB2, STAT5b, while the expression of miR-199а-3p/214 was lowered that resulted in the higher expression of the COX2 protein. The myomatous tissue was characterized by the higher expression of let-7 and the lower expression of miR-200a, miR-200b, and some other miRNAs that significantly altered the expression of targeted proteins HMGA2, ZEB1, ZEB2, CYP1B1, VEGFA, CDH1, and others [50].
It appears that the benign proliferative diseases of the female reproductive system share the same epigenetic mechanisms involving miRNAs (let-7, miR-200, miR-199, miR-145, and others) and their targeted proteins (HMGA2, COX2, and others). These miRNAs exert their biological effects through the control of the transcription of some genes (including the syntropic ones) and respective targeted proteins. On the other hand, the differences in the epigenetic landscape may explain the existence of different clinical forms of the disorders as well as their unique clinical manifestation.
Conclusion. In summary, the commonly observed comorbidity of the benign proliferative diseases of the female reproductive system (uterine leiomyoma, endometriosis, endometrial hyperplasia) is based on the shared risk factors and mechanisms of the pathogenesis; the latter include, among the others, epigenetic factors and syntropic genes. Identification of the shared factors for these diseases helps to determine therapeutic targets and develop an efficient treatment strategy against comorbidity of the disorders.
Financial support
No financial support has been provided for this work.





















Список литературы
Список использованной литературы появится позже.