In vitro activity of the novel antimicrobial peptide WR-286 in combination with vancomycin against methicillin-resistant
Staphylococcus aureus
Aннотация
Background: Multidrug resistance bacteria are a serious problem for health specialists and all the people in the world. The main reasons for this problem are the misuse of antibiotics and the limited number of antibiotics as compared to different human diseases. The important antibiotic-resistant bacteria include Methicillin-resistant Staphylococcus aureus (MRSA). Antimicrobial peptides (AMPs) are regarded as promising antimicrobial agents because they satisfy the requirements for the creation of innovative antimicrobial drugs. The aim of the study:To design a novel antimicrobial peptide and study its effect against MRSA when we combined it with vancomycin. Materials and methods: To produce the hexapeptide WR-286, 1-(2, 6-difluorobenzyl)-1H-1, 2, 3-triazole-4-carboxylic acid, tryptophan (W), and arginine (R) were rationally combined. Different bacterial strains were used to test WR-286's antibacterial properties. Investigations of WR-286's hemolytic activity toward human erythrocytes were also conducted. Finally, utilizing the checkerboard approach and the fractional inhibitory index, synergistic tests with vancomycin were carried out. Results: With MIC values as low as 35 μM, WR-286 demonstrated strong antibacterial activity against MRSA. The toxicity of WR-286 to human red blood cells was very low. Additionally, the peptide and vancomycin's activity were improved by the synergistic experiments. Conclusion: According to the current investigation, WR-286 has little hemolytic activity and displays promising antibacterial activity against MRSA. Additionally, when coupled with vancomycin, the peptide has synergistic effect. The novel AMPs described in this work are promising potential candidates for antimicrobial drug development
Ключевые слова: antimicrobial peptide, WR-286, minimum inhibitory concentration, MRSA, vancomycin, antibiotic resistance
К сожалению, текст статьи доступен только на Английском
Introduction. Staphylococcus aureus is a type of bacteria that is commonly found on the skin and in the nose of healthy people. However, it can cause serious medical problems if it enters the body through a cut or wound [1]. This is called methicillin-resistant staphylococcus aureus (MRSA). In 2013, more than 65,000 cases of MRSA were reported in the United States. Many healthcare workers develop MRSA infections on the job [2]. Fortunately, there are steps we can take to lower the risk of transmission to the general public. MRSA is resistant to many common antibiotics, making it difficult to treat. People with MRSA infections are at an increased risk for developing life-threatening conditions such as bloodstream infection, sepsis and cancer [3]. Additionally, MRSA can spread easily among people in hospitals and other healthcare facilities. This is because healthcare workers and other patients who are sicker than average frequently contact things with which they have been infected. Unfortunately, many of these people don't recognize that they have MRSA infections when they return home. This allows the bacteria to spread further and cause more severe health issues among susceptible individuals [4].
Therefore, to overcome this problem and due to the increasing resistance of MRSA, various studies have been conducted, one of which is the combination of vancomycin with antimicrobial peptides (AMPs) [5, 6].
Due to their many benefits, including their broad-spectrum and quick killing mechanism against a wide variety of bacteria, AMPs are regarded as a great alternative to conventional antibiotics. They also show potential against germs that are multi-drug resistant. Furthermore, because of their extensive structural variety and non-specific interactions with bacterial components, they have a low likelihood of causing microbial resistance [7].
Vancomycin is a drug used to treat various infections. It is a member of the teicoplanin family, which includes other antibiotics such as gentamicin and daptomycin [8]. Vancomycin is effective against a variety of bacteria, including those that are resistant to other antibiotics like MRSA. But vancomycin has many side effects like nephrotoxicity and ototoxicity that limit their use [9].
In this study we designed a novel antimicrobial peptide and combined it with vancomycin and evaluated the activity of this combination against MRSA.
Materials and method
Peptide design and synthesis
WR-286 was rationally designed by incorporating alternating subunits of both arginine (R) and tryptophan (W) to create an ultrashort hexapeptide. The peptide was further conjugated with 1-(2, 6-difluorobenzyl)-1H-1, 2, 3-triazole-4-carboxylic acid. WR-286 was synthesized using conventional solid-phase Fmoc chemistry (GL Biochem, Shanghai, China). WR-286 was synthesized following standard Fmoc solid-phase protocols on Wang resin. Peptide elongation was effected using standard HBTU coupling chemistry in dimethylformamide (DMF) solvent with a fourfold molar excess of diisopropyl ethylamine (DIEA) in N-methyl-2-pyrrolidone (NMP) and a threefold molar excess of each Fmoc-protected amino acid or 1-(2, 6-difluorobenzyl)-1H-1, 2, 3-triazole-4-carboxylic acid. WR-286 was cleaved from the resin, using 95% trifluoroacetic acid (TFA), 2.5% triisopropylsilane, and 2.5% thioanisole (3 h, room temperature), and precipitated using cold (−20 ◦C) diethyl ether. The synthesized peptide’s purity was determined by reverse-phase high-performance liquid chromatography (RP-HPLC). The identification of WR-286 was confirmed by mass analysis and through the employment of electrospray ionization mass spectrometry (ESI-MS) [10, 11, 12].
Determination of the Active Peptides by Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs)
The Clinical and Laboratory Standards Institute (CLSI) criteria were followed to assess the MIC and MBC of the active peptides using sterile 96-well polypropylene microtiter plates. In a nutshell, the MHB was taken out of the stock media of frozen glycerol and used as the growth medium for organisms. Before use, bacterial cells were diluted to 106 CFU/ml in the same medium after being cultured overnight in Muller Hinton Broth. As final concentrations, several dilutions with concentrations between 0.5 and 100 μM were created. Then, 50 μL of the peptide concentration and 50 μL of diluted bacterial solution were added into each well of 96-well microtiter plates. At 37 Co, the plate was incubated for 18 hours. Next, using an ELISA plate reader to measure OD at 570 nm to assess the rate of bacterial growth, MIC was calculated (as the lowest concentration of antimicrobial drugs which is needed to inhibit the growth of the bacteria). Each plate included a positive control column (50 μl of bacterial suspension plus 50 μl MHB without any antimicrobial agents) and a negative control column (100 μl of MHB in each well) to ensure the activity of bacteria and the sterility of MHB respectively and repeated three times [13, 14, 15].
For MBC determination, 10 µl each of clear negative wells and turbid positive control wells were taken, seeded on sterile labeled agar with nutrient medium, and incubated for 24 hours at 37oC.
The lowest concentration that led to having < 0.1% viable cells (killing 99.9 %) was referred to as the MBC value.
MIC Determination of Peptide-Vancomycin Combination
According to the broth microdilution checkerboard technique, MICs of peptide-vancomycin combination against stander bacterial strains of S.areues and MRSA. However, in this assay, each microtiter well contained a mixture of WR-268 peptide and vancomycin in different concentrations. 25 µl of each peptide concentration and 25 µl of vancomycin (from 0.25 to 200 μM) were added to six wells of a sterile flat–bottomed 96 well-plate that contained 50 µl of the diluted bacterial suspension. MICs determination made in triplicate [16, 17, 18].
Determination of Synergism using Fractional Inhibitory Concentration
The fractional inhibitory concentration (FIC) is the summation of the inhibitory concentration values of each component resulted in the antimicrobial combination divided by the inhibitory concentration alone [19-22].
The FIC indices were interpreted as follows:
≤ 0.5: synergistic activity, 0.5-1: additive activity, 1-4 indifferent, >4: antagonistic. Interpretation and assessment of the FIC index and antimicrobial activity of peptides-antibiotics combinations were conducted according to the broth microdilution checkerboard technique
Determination of the ability of the designed peptide to cause hemolysis to human erythrocyte, hemolytic assays was performed. Two ml of human blood was placed into a 50-ml centrifuge tube, centrifuged at 3000 xg for 5 min. The supernatant was discarded and the cell pellet was suspended in 48 ml of PBS and centrifuged at 3000 xg for 5 min; this step was repeated three times. Finally, the cell pellet was re-suspended in a sterile tube containing 50 ml PBS to reach a final concentration of 4% RBC and PBS containing different concentrations of the peptides. Then 1 ml of each concentration was added to 1 ml of erythrocyte suspension [23].
Controls were prepared by the addition of 5 µl of Triton X-100 to 1 ml of RBC suspension (positive control). The blank (negative control) was prepared by adding 1 ml of RBC suspension with PBS. The suspension was incubated for 60 min at 37 °C. Tubes were gently vortexed and 1 ml of each sample was aspirated and placed into sterilized Eppendorf tubes and then centrifuged for 5 min at 3000 xg. From each supernatant 100ul. Was placed into a well of a 96-well plate. Absorbance was measured at λ= 570 nm with the aid of the Absorbance Microplate Reader. The percentage of hemolysis was calculated according to the following equation [24].
% Hemolysis =  × 100
× 100
Where A: is OD 450 with the peptide solution,
A0: is OD 450 of the blank.
And AX: is OD 450 of control (0.1% Triton X-100).
Results
The synthesis of WR-286 peptide
As displayed in Fig. 1, the peptide was designed using three subunits of arginine and three units of tryptophan in combination with 1-(2, 6-difluorobenzyl)-1H-1, 2, 3-triazole-4-carboxylic acid. Tryptophan was integrated as hydrophobic moiety, and because of its membrane interface interaction. It exhibits a strong preference when compared to the other hydrophobic amino acids. 1-(2, 6-difluorobenzyl)-1H-1, 2, 3-triazole-4-carboxylic acid was used due its hydrophobicity nature and having some antimicrobial effect.

Bacterial Susceptibility Assay of the WR-286 peptide and vancomycin
The peptide and vancomycin were tested against control and resistance strain of S. aureus (ATCC 29215) and MRSA (ATCC BAA-41). The results showed that the peptide has a good activity against both strains with MIC value 25 and 35 μM against S. aureus (ATCC 29215) and MRSA (ATCC BAA-41) respectively with bactericidal mode of action =. The Vancomycin has MIC value of 0.5 μM against controlled strain and 2 μM against MRSA. The results of MIC and MBC were shown in Table 1.
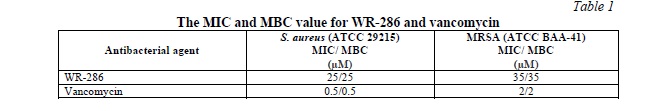
Study the synergistic effect of the peptide combination with vancomycin
WR-286 and Vancomycin synergistic effects were evaluated utilizing the checkerboard method technique. Table 2 displays the synergy values for WR-286 and Vancomycin as calculated by the FIC index.
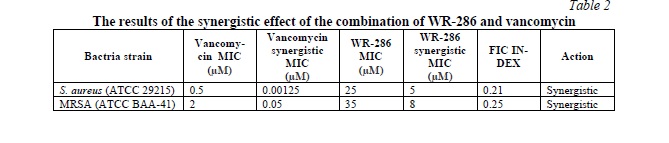
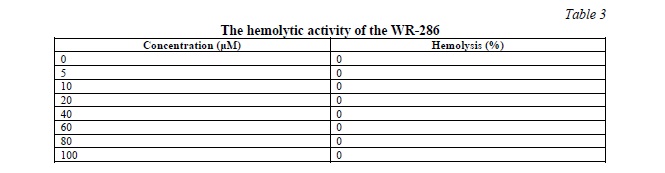
Hemolytic activity of WR-286 peptide
Up to a concentration of 100µL, WR-286 has no hemolytic action against human erythrocytes. The results of hemolytic assay were shown in Table 3.
Discussion. Antibiotic resistance occurs when microorganisms become unaffected by antimicrobial agents. Accordingly, the drug becomes ineffective and infections persist in the body, increasing the risk of spread to others. In 2014, the World Health Organisation (WHO) published a new report on the problem of bacterial resistance, which now exists in all regions of the world and has the potential to affect anyone, no matter who they are [25, 26].
The effective treatment and prevention of an increasing range of infections are seriously threatened by antimicrobial resistance (AMR) caused by bacteria, parasites, viruses, and fungi. Using effective antimicrobial agents increases the success rate of major surgeries and chemotherapy otherwise, they would be compromised [27]. Also, the AMR exerts major negative feedbacks to global public health that needs actions across society and all government sectors. From an economic point of view, patients with resistant infections need a high cost for treatment in comparison with other patients [28]. According to a recent WHO report on the onset of bacterial resistance, the most type of bacteria causing the major types of resistant nosocomial infections is Staphylococcus aureus. S. aureus is the strain that develops MRSA by interspecies transfer of the mecA gene from an ancestral Staphylococcus species to S. aureus mediated by a special staphylococcal mobile genetic element [29].
MRSA is a Gram-positive bacterium that causes a wide range of very dangerous human diseases. Nosocomial infections caused by MRSA strains have become a major problem internationally as MRSA, by causing invasive infection, leads to an increase in mortality of up to 20%.
It causes inflammation in many tissues, septicemia, and even life-threatening infections. The risk of MRSA infections comes not only from the emergence of MDR but also from the ability of bacteria to develop strong biofilms structures [30].
MRSA strains are responsible for many critical hospital and community-acquired infections. According to the National Nosocomial Infections Surveillance System, the incidence of MRSA infection is rising and 60% of intensive care unit admissions were accounted for MRSA infection. Treating these infections is more challenging with the traditional antibiotic. MRSA exhibits resistance to a wide range of antibiotics, including second-and third-line drugs. Recently, it has been suggested that a combined synergistic approach will delay or eliminate the development of drug resistance, reduce the doses of individual drugs and hence reduce side effects.
Many studies assessed the outcomes of Host Defense Peptides (HDPs) or new synthesized AMPs and conventional antibiotics in combinations against various multidrug-resistant bacteria (MDRB) [31]. These studies reported synergistic activities or an antibacterial activity improvement using many combinations. As AMPs are considered as membrane targeting agents that distort bacterial cell membranes via pore formation mechanisms and lead to an increase in the permeability of cell membrane, this consequently would lead to an increase of antibiotics entry into the cell to accomplish the damage process more efficiently and rapidly. The WR-286 peptide was synthesised using three units of the amino acid arginine to provide sufficient electrostatic attraction with the negative head groups of the phospholipids of the bacterial cell membrane.
Our results are consistent with previous studies showing that membrane disruption depends on the cationic residue and, therefore, the positive charge should depend on arginine rather than lysine or ornithine, especially if the peptide consists of less than three residues [32].
This phenomenon explains the role of arginine's guanidine moiety that is responsible for increasing the binding of the cationic moiety in AMPs to the membrane surface via forming a complex with the phosphate groups belonging to the membrane phospholipid bilayer. Tryptophan was used to render the peptide hydrophobicity, which plays a significant role in enhancing peptide-membrane interaction compared to other hydrophobic amino acids. It has been reported that a high propensity for membrane insertion happens as a consequence of the specific affinity between the indole group of tryptophan and carbonyl moieties of phospholipids and hence membrane disruption [33].
As shown in our study, the results of the MIC and MBC indicate that our peptide showed a good activity against MRSA with MIC value of 35 µM with bactericidal mode of action. In addition, WR-286 has no haemolytic activity against erythrocytes, the low haemolytic activity is due to the increase in cationic charge to approximately +3, which helps to minimise haemolytic toxicity against erythrocytes.Accordingly, mass charge plays a major role in creating a sufficient electrostatic attraction and hence targeting the negative head groups of the bacteria cell membrane with very negligible toxic effects toward human erythrocytes. Several studies have reported that combining AMPs with antibiotics will provide enhanced microbiocidal activity for both the peptide and the antibiotic [34]. In this study we compared WR-286 with vancomycin to see the activity of this combination against MRSA. Vancomycin does not work intracellularly but functions by inhibiting cell wall synthesis by binding to the D-Ala-D-Ala terminal of the growing peptide chain during cell wall synthesis, resulting in inhibition of the trans peptidase, which prevents further elongation and cross-linking of the peptidoglycan matrix. It displayed a synergistic effect against Gram-positive bacteria. This could be explained by the fact that vancomycin acts against the cell wall, facilitating the penetration of peptides to their target sites in the cell membrane, which ultimately leads to rapid cell lysis and a decrease in the effective concentrations required to inhibit bacterial growth exhibited by the peptide and vancomycin [35].According to the synergistic mode of action exhibited when combing WR-286 with vancomycin, this dramatic decrease in peptide concentrations needed to kill bacteria efficiently while displaying negligible cytotoxic effects could offer a great strategy for AMP development into effective therapeutics with the current challenge faced globally by the lack and discovery of new safe and effective antibiotics [36].
Conclusion. A new conjugated ultrashort antimicrobial peptide with high activities against MRSA and low hemolytic activities is designed, and its antimicrobial characteristics are reported. When paired with vancomycin, the peptide showed synergistic effects, making it a strong option for future antimicrobial research.
Financial support
No financial support has been provided for this work.





















Список литературы
Список использованной литературы появится позже.