Differential expression of CYP19A1 and lncRNA-CTBP1-AS in the granulosa cells of women with polycystic ovary syndrome
Aннотация
Background: Hyperandrogenism is the basic indicator of polycystic ovarian syndrome (PCOS) and a key factor in its pathological processes. Patients with PCOS have higher testosterone levels along with a decline in the estradiol/testosterone ratio, which may indicate an aromatase dysfunction. The aim of the study:Investigating the expression levels of CYP19A1 and lncRNA-CTBP1-AS in the granulosa cells (GCs) of women with and without PCOS. Materials and methods: We compared the expression levels of CYP19A1 and lncRNA-CTBP1-AS in granulosa cells from 16 PCOS patients and 20 controls who underwent assisted reproductive technology (ART) treatment at the Center for Human Reproduction and IVF (Rostov-on-Don, Russia) using quantitative real-time PCR. Results: We observed a significant downregulation of CYP19A1 expression in the GCs of PCOS patients, with expression levels approximately 4.7 times lower compared to the control group. Furthermore, we found a positive correlation between CYP19A1 expression and the E2/T ratio in PCOS patients. Conversely, PCOS patients exhibited a remarkable upregulation of CTBP1-AS expression in their GCs, with levels up to 23 times higher than those in the control group. Importantly, we also identified a significant negative correlation between the expression level of CTBP1-AS and CYP19A1 in the GCs of PCOS patients. Conclusion: Our results indicate a potential regulatory role of CTBP1-AS in CYP19A1 expression and suggest its involvement in the pathogenesis of PCOS
К сожалению, текст статьи доступен только на Английском
Introduction. Polycystic ovarian syndrome (PCOS) is a common multifactorial endocrinological disorder, that affects around 4 to 20% of women aged 18 to 44 years [1]. It is thought to be the primary contributor to female infertility due to anovulation [2]. PCOS is highly heterogeneous and diagnosed by the presence of at least two of the following three criteria: hyperandrogenism, the polycystic morphology of the ovaries, and oligo- or anovulation. In PCOS, the steroid hormone imbalance and high levels of the luteinizing hormone (LH) lead to folliculogenesis disturbance and anovulation [3, 4]. During ovarian steroidogenesis, androstenedione and testosterone are synthesized in theca cells by CYP11A1, CYP17A1, and HSD17B, which are then converted to estrone and estradiol, respectively, by aromatase in granulosa cells (GCs) under FSH stimulation [5, 6]. Blood serum and follicular fluid (FF) testosterone and androstenedione levels are higher in PCOS patients compared to non-PCOS women, whereas the estradiol-to testosterone E2/T ratio is lower [7, 8, 9], which may indicate a defect in the estrogen biosynthesis mechanism, i.e., aromatase dysfunction.
Aromatase is the main enzyme responsible for the irreversible conversion of androstenedione and testosterone into estrogen and estradiol, respectively. It is encoded solely by the CYP19A1 gene, which is located at ch15 q21.2 [10] and is mainly expressed in the ovaries, placenta, testis, adipose tissue, and brain. CYP19A1 gene is regulated by a complex mechanism, the regulatory region is about 93 kb with more than 10 promoters that are activated differently in a tissue-specific manner [11]. Among these promoters, PII has been shown to be an essential promoter in GCs, and P I.3 has been identified as a PII enhancer [12].
Recently, studying the role of lncRNAs in transcriptional and post-transcriptional modifications has attracted increasing attention. Dysregulated lncRNAs are involved in various diseases, such as cancer [13, 14], diabetes mellitus [15], cardiovascular diseases [16] and reproductive disorders [17, 18]. While several lncRNAs with altered levels have been observed in plasma, follicular fluid (FF), and granulosa cells (GCs) of patients with PCOS [19-22], the full understanding of their pathological mechanisms remains elusive.
LncRNA c-terminal binding protein 1 antisense (CTBP1-AS) – an androgen receptor modulator – localizes in the antisense (AS) region of CTBP1 in chr4 p16.3. CTBP1 consists of 10 exons, while CTBP1-AS consists of 2 exons. Both are expressed in almost all tissues. But the expression of CTBP1-AS is much lower, according to the UCSC genome browser https://genome-euro.ucsc.edu/. Previous studies have revealed the involvement of CTBP1-AS in the regulation of androgen metabolism and its association with the androgen receptor (AR) signaling pathway in prostate cancer [23]. Furthermore, other research has identified CTBP1 as a transcriptional regulator of CYP19 in prostate cancer [24]. Moreover, a recent study has shown overexpression of CTBP1-AS in breast cancer [25]. But data on the functional importance of the lncRNA CTBP1-AS and its role in the pathological mechanisms of these diseases are sparse.
The aim of the study. Building upon these findings, we hypothesize that CTBP1-AS, through its potential interaction with CYP19, may play a role in modulating androgen and estrogen balance and the regulation of androgen metabolism in the granulosa cells of women with PCOS. Therefore, the purpose of this study is to investigate the expression levels of CTBP1-AS and CYP19A1 in granulosa cells of women with PCOS and explore the potential correlation between their expression patterns.
Materials and methods
Study subjects
36 women aged (22-40) years were enrolled in this case-control study. 16 PCOS patients and 20 controls underwent assisted reproductive technologies (ART) treatment at the Center for Human Reproduction and IVF (Rostov-on-Don, Russia), in 2021-2022. PCOS diagnosis was done according to Rotterdam criteria, Patients were diagnosed when they had at least two of the following three criteria: 1) hyperandrogenism confirmed clinically or biochemically; 2) oligo- or anovulation; 3) the polycystic ovarian morphology confirmed by ultrasound with the presence of > 12 follicles (2-9 mm in diameter) in each ovary, or 20 antral follicles in both ovaries, or an enlarged ovary with a volume > 10 cm3. The control group consists of women who underwent IVF/ICSI due to tubal factor infertility or male infertility. Endocrinological pathologies, such as hyperprolactinemia, Cushing’s disease, adrenal hyperplasia, or ovarian tumors, were excluded from both groups.
Blood serum, follicular fluid and granulosa cells collection
Morning blood samples were collected after a fasting period of 12-14 hours. After centrifugation at 3000 rpm for 5 minutes, the serum was separated from the blood samples and stored at -20°C until further analysis. Additionally, follicular fluid samples were aspirated during oocyte aspiration. FF without red blood cells contamination was centrifuged at 3000 rpm for 5 minutes and stored at -20°C until assayed. The granulosa cell samples were collected from follicle fluid in RNA stabilizing solution “IntactRNA” from (Evrogen, Russia) and stored at -80 °C.
The hormonal analysis
Biochemical analyses were conducted on blood samples and follicular fluid samples from 16 PCOS patients and 20 controls. The levels of FSH, LH, estradiol, AMH, and insulin were measured using the Beckman Coulter test systems on the Access 2 automatic analyzer. The total testosterone, DHEA-S, and SHBG levels were quantified using the (Alkor-Bio test systems, Russia) on an Alisey QS automatic immunochemical analyzer. For the measurement of DHEA, the (DBC test system, Russia), was utilized on the same analyzer. Fasting blood plasma glucose was determined using a LabSystem analyzer from Finland, with reagents sourced from Biocon in Germany. The HOMA-IR index was calculated using the formula: HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L) / 22.5.
Quantitative real-time PCR
The total RNA was extracted from GCs using the “ExtractRNA” kit from (Evrogen, Russia). Synthesis of cDNA from the total RNA was carried out using the reverse transcription kit “MMLV RT kit” from (Evrogen, Russia). Following the manufacturer's instructions, a total of 20 μl reaction solution containing 6 μl RNA, 1 μl of random primer, and 0.5 μl MMLV revertase. The reaction mixture was prepared using RNase-free water. Then the samples were incubated at 40 °C for 40 min, and finally, to stop the reaction, the mixture was incubated at 70 °C for 10 min.
Real time-PCR was performed to measure the relative expression of CYP19A1 and CTBP1-AS in GCs using the SYBR Green master mix “5X qPCRmix-HS SYBR” from (Evrogen, Russia) and the QuantStudio Real-Time PCR Systems (Applied Biosystems, Thermo Fisher Scientific, United States). The 25 μl PCR reaction solution contains 5 μl (1x) qPCRmix-HS SYBR, 1 μl of each primer forward and reverse primers, 2 μl cDNA templates and 16 μl Nuclease-Free Water. PCR conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 10 s, and 72 °C for 20 s. This was followed by high-resolution melting curves of PCR amplicons with temperatures ranging from 60 °C to 95 °C. As internal control, we used GAPDH. All samples were conducted in duplicate. Fold changes were calculated using the 2-ΔΔCt method. The sequences of the primers were as follows: CYP19A1, forward primer 5′- GAGAATTCATGCGAGTCTGGA -3′; reverse primer, 5′- CATTATGTGGAACATACTTGAGGACT -3′. GAPDH, forward primer 5′- GGGAAACTGTGGCGTGAT -3′; reverse primer 5′- GAGTGGGTGTCGCTGTTGA -3′. CTBP1-AS, forward primer, 5′- ACAACACAAAGCCCCGGAA -3′; reverse primer, 5′- AGTGAAGAATGGTCTCGCCC -3′.
Statistical analysis
We used GraphPad Prism 7 for our statistical analysis. The comparisons of variables were carried out using a two-tailed unpaired t-test or Unpaired t test with Welch's correction, as appropriate. Correlation analyses were performed using a two-tailed Pearson’s correlation coefficient test. The variables were expressed as means ± SEM for each group. Statistical significance was assessed at p < 0.05.
Results
Hormonal profiles in blood serum of PCOS patients and controls
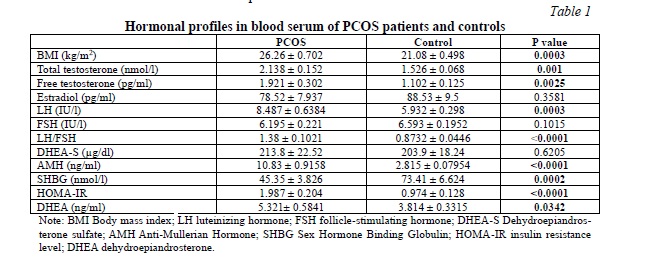
Table 1 presents the hormonal characteristics of individuals with PCOS and healthy controls. To assess the androgen profile, we measured total and free testosterone, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEA-S) in both groups. As anticipated, the PCOS group exhibited significantly higher levels of serum total and free testosterone and DHEA compared to the control group. However, the difference in DHEA-S levels between PCOS patients and controls was not statistically significant. Notably, LH, LH/FSH, anti-mullerian hormone (AMH), and BMI were significantly elevated in the PCOS group. Additionally, PCOS patients demonstrated a significant increase in HOMA-IR levels, while SHBG levels were significantly lower. Conversely, no significant differences were observed in FSH and estradiol levels between the two groups.
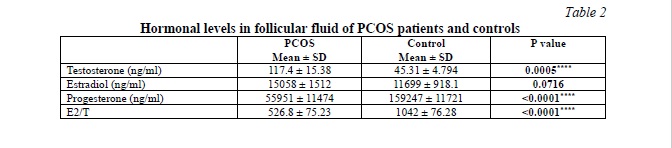
Follicular fluid hormones profile in PCOS patients and controls
We evaluated the differences in testosterone, estradiol, and progesterone levels in the FF between PCOS patients and the control group. The data are presented in Table 2, Fig. 1. Our results showed that PCOS patients had significantly higher testosterone levels (p = 0.0005) and lower progesterone levels (p < 0.0001) in comparison with the control. While the increase in the FF estradiol levels of PCOS patients is not significant (p = 0.0716). However, the E2/T ratio showed a significant difference between both groups (p < 0.0001). It was about two times lower in PCOS patients compared to the control group, with a difference between means (515 ± 111.4).
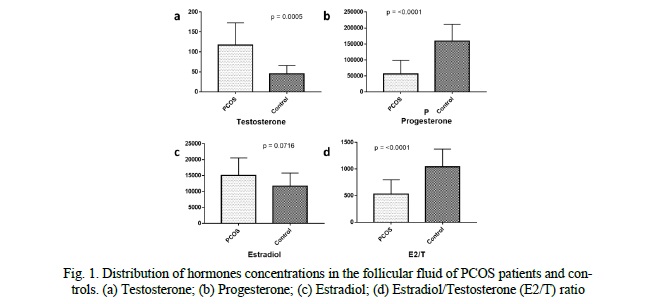
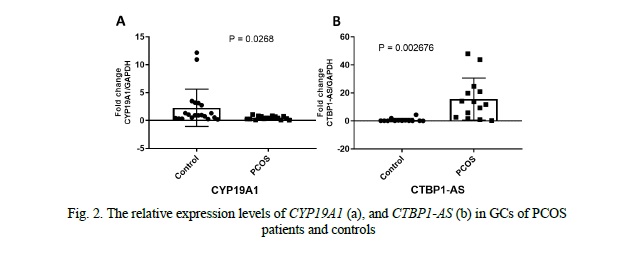
CYP19A1 and CTBP1-AS expression levels in granulosa cells
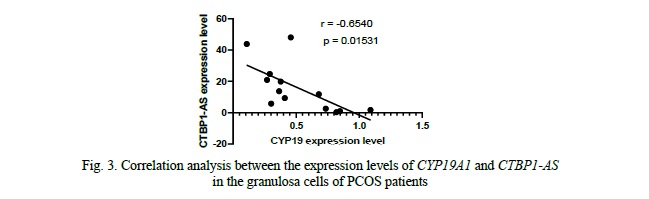
We compared the expression levels of CYP19A1 and CTBP1-AS in GCs of 16 PCOS patients and 20 healthy controls. We found that the mRNA levels of CYP19A1 were significantly decreased by approximately 4.7-fold in PCOS patients' GCs compared to healthy controls (p = 0.0268, Fig. 2). Conversely, the expression level of CTBP1-AS was found to be predominantly low in GCs of healthy women, with undetectable levels observed in 8 samples, while in PCOS patients, the expression level of CTBP1-AS was significantly elevated, up to 23-fold higher compared to the control group (p = 0.002676, Fig. 2). Furthermore, when exploring the correlation between CYP19A1 and CTBP1-AS expression, a negative correlation was observed (r = -0.6540), reaching statistical significance (p = 0.01531, Fig. 3). These results suggest that the dysregulation of CTBP1-AS may play a role in the aberrant expression of CYP19A1 in PCOS, highlighting its potential involvement in the pathogenesis of PCOS.
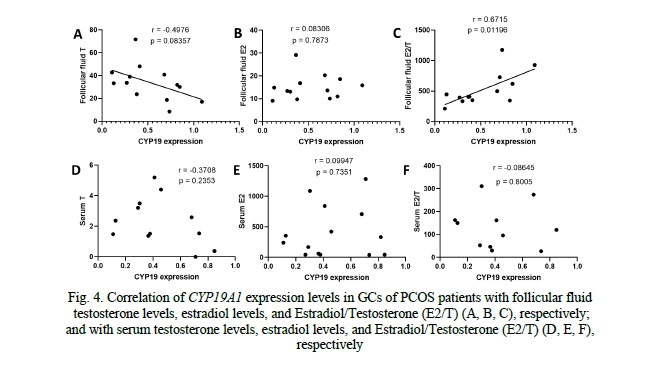
Correlation of CYP19A1 and CTBP1-AS expression levels with hormone profiles in serum and follicular fluid of PCOS patients
Analysis of the correlation between CYP19A1 expression levels in granulosa cells (GCs) of PCOS patients and follicular fluid hormones revealed a negative correlation trend with testosterone (r = -0.4976, p = 0.083), indicating that higher CYP19A1 expression was associated with lower testosterone levels. However, this correlation did not reach statistical significance. No significant correlation was observed between CYP19A1 expression and estradiol (r = 0.08306, p = 0.7873). Regarding the estradiol-to-testosterone ratio (E2/T), we observed a significant positive correlation with CYP19A1 expression in GCs of PCOS patients (r = 0.8715, p = 0.01196), indicating that decreased CYP19A1 expression levels were associated with an altered E2/T ratio) (Fig. 4). However, no significant correlations were found between CYP19A1 expression and serum hormone levels, including testosterone (r = -0.3708, p = 0.2353), estradiol (r = 0.09947, p = 0.7351), or E2/T ratio (r = -0.08645, p = 0.8005) (Fig. 4).
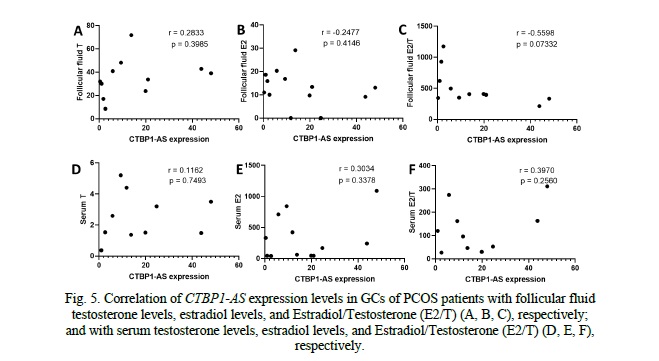
When examining the correlation between CTBP1-AS expression levels in granulosa cells (GCs) of PCOS patients and follicular fluid hormones, no significant correlations were observed (Fig. 5). The correlation coefficients for testosterone (r = 0.08616, p = 0.7901), estradiol (r = -0.03984, p = 0.8924), and the estradiol-to-testosterone ratio (E2/T) (r = -0.2964, p = 0.3462) were all close to zero, indicating a lack of association between CTBP1-AS expression and these hormone levels in follicular fluid. Similarly, when investigating the correlation between CTBP1-AS expression levels in GCs of PCOS patients and serum hormone levels, no significant correlations were found (Fig. 5). These findings indicate that CTBP1-AS expression in GCs may not directly influence the androgen metabolism and estrogen biosynthesis.
Discussion. Polycystic ovary syndrome is a highly heterogeneous disease. Hyperandrogenism is the main diagnostic criterion of PCOS with an incidence rate of 60-80% [5] and plays an important role in its pathological mechanisms. PCOS patients have increased levels of androstenedione and testosterone in the serum, and dehydroepiandrosterone (DHEA) is also elevated in up to 50% of hyperandrogenic women [26]. It is believed that these circulating androgens are from ovarian sources. It has been shown that there is a subgroup of PCOS patients with elevated levels of testosterone in the FF, despite its normal circulating levels [8]. This hormonal imbalance has a detrimental impact on ovarian function leads to theca cell hyperplasia, degeneration of GCs, arrest of follicular development at the small antral stage, and anovulation [27]. GCs in polycystic ovaries fail to increase the expression of aromatase, which reduces its capacity to synthesize estrogens. Moreover, evidence shows that polycystic ovaries have an increased capacity to secrete androgens [7]. In PCOS, excessive levels of androgens and a decrease in the E2/T ratio indicate a disruption of androgen metabolism and estrogen biosynthesis, which may indicate insufficient enzymatic activity of aromatase, and this might be due to reduced expression of CYP19A1 gene, or the presence of an aromatase inhibitor. Our results showed a decrease in CYP19A1 gene expression in GCs of PCOS patients by 4.7 times compared to the control group. which is consistent with a study conducted in Indonesia on patients with PCOS. This study showed that the relative level of aromatase mRNA expression in GCs was reduced by 2.6 times in PCOS women with compared to the control [28]. The expression levels of CYP19A1 in granulosa cells (GCs) of PCOS patients exhibited a negative correlation with testosterone levels and a positive correlation with the E2/T ratio in follicular fluid (FF). However, no significant correlations were observed between CYP19A1 expression levels and testosterone, estradiol, and E2/T ratio in the blood serum of PCOS patients. This lack of significant correlation suggests that the association between CYP19A1 expression and hormone levels may be more pronounced within the local follicular fluid environment. CYP19A1 is primarily expressed in the granulosa cells of ovarian follicles and is involved in the conversion of androgens to estrogens. Therefore, the local correlation between CYP19A1 expression and follicular fluid hormones may be more evident compared to the systemic circulation of serum hormones. It is also possible that the effects of CYP19A1 expression on hormone levels are more pronounced at the local level within the follicular microenvironment, potentially influenced by factors specific to the ovarian follicles such as paracrine signaling, local concentrations of factors involved in ovarian steroidogenesis, or feedback mechanisms.
CYP19A1 gene spans a region of about 123 kb, including 30 kb coding region and a 93 kb regulatory region with more than 10 proximal and distal promoters [10, 12]. Each of them is regulated by a specific set of transcription factors, cytokines, or hormones. Although the expression of the CYP19A1 gene is regulated differently by a complex mechanism in a tissue-specific manner. It encodes the same protein with an identical open reading frame from exon II to X [12]. It has been shown that the ovary-specific promoter (PII) is less active in PCOS GCs. Agarwal and his colleagues suggested that aromatase activity might be regulated locally by aromatase inhibitor compounds in the FF of PCOS patients [29]. According to these data, the expression of 5D-reductase is increased in GCs of PCOS patients, inhibit the aromatase activity competitively by blocking its active sites. CYP19A1 gene expression can be affected by epigenetic modifications in the regulatory regions. The epigenetic markers including H3K9 di-methylation and H3K9 acetylation as repression and activation markers, respectively, have been studied in the chromatin of PCOS cumulus cells at the proximal promoters of CYP19A1 (PII and PI.3) [30]. Significant differences in these markers have been shown in PCOS cumulus cells compared to the control, highlighting the crucial role of epigenetic mechanisms in PCOS development [30]. Recent research has focused on investigating the involvement of long non-coding RNAs (lncRNAs) in the pathological mechanisms of PCOS, revealing the upregulation of lncRNA HUPCOS in GCs of PCOS patients. This upregulated lncRNA is associated with hyperandrogenism and androgen excess by inhibiting aromatase expression in GCs [31]. Our results revealed a substantial increase in CTBP1-AS gene expression by 23 times in GCs of PCOS patients compared to controls. Notably, CTBP1-AS gene expression exhibited a negative correlation with CYP19A1 gene expression in PCOS patients' GCs, suggesting a potential inhibitory role of CTBP1-AS in CYP19A1 expression and its involvement in hyperandrogenism and PCOS pathogenesis. Moreover, studies conducted on Chinese and Indian populations have linked elevated CTBP1-AS expression in serum with PCOS, emphasizing its correlation with androgenic effects and total testosterone levels [32, 33]. However, in our investigation, no significant correlations were observed between CTBP1-AS expression levels in GCs of PCOS patients and the levels of testosterone, estradiol, and E2/T ratio in blood serum or follicular fluid (FF). These findings indicate that CTBP1-AS expression in GCs may not directly influence androgen metabolism and estrogen biosynthesis. It is plausible that additional factors or regulatory mechanisms are involved in modulating steroidogenesis in PCOS patients. Further investigations are necessary to unveil the underlying molecular mechanisms driving these associations and better understand the complex interplay between CTBP1-AS, hormone levels, and PCOS pathophysiology.
Conclusion. Our study revealed significant dysregulation of CYP19A1 expression in granulosa cells (GCs) of PCOS patients compared to the control group. This dysregulation was associated with altered hormone levels specifically within the follicular fluid (FF). We also observed an upregulation of CTBP1-AS in PCOS patients' GCs, and interestingly, this upregulation showed a negative correlation with CYP19A1 expression. These findings highlight the potential role of CTBP1-AS in modulating CYP19A1 expression and suggest its involvement in the pathogenesis of PCOS, particularly in relation to hyperandrogenism.
Financial support
This study was funded by the Russian Science Foundation (RSF) grant № 23-15-00464.





















Список литературы
Список использованной литературы появится позже.