Bioactivity of Vitamin D and Tinospora cordifolia Extract in Increasing Cathelicidin Synthesis and Regulating TNF Alpha Production in CD66a Cells
Aннотация
Background: Cathelicidin is an antimicrobial peptide that plays an important function in the innate immune system as the first line of defense against infections. There is still not a lot of information on the compounds that keep the body's cathelicidin production going. The aim of the study:To observe the effect of vitamin D and Tinospora cordifolia Extract (TCE) for 28 days on the production of cathelicidin and (Tumor necrosis Factor) TNF alpha in CD66a cells shortly after the mice were infected with E. coli. Materials and methods: We conducted a study with five treatment groups using BALB/c mice. Normal Group (NG): standard diet without treatment, Infected Group (IG): standard diet and infected on day 29, Vitamin D Group (VDG): standard diet + Vitamin D 100 micrograms/kg body weight and infected on day 29, Tinospora cordifolia Group (TCG): standard diet + TCE 100 mg/kg body weight and infected on day 29, and Combination group (CG): standard diet+ Vitamin D 100 micrograms/kg body weight + TCE 100 mg/kg body weight and infected on day 29. On day 29, the spleen was isolated at the end of treatment, and the production of cathelicidin and TNF alpha in CD66a cells was evaluated using flow cytometry and analyzed using one-way ANOVA (p < 0.05). Results: Our findings show that vitamin D significantly increases cathelicidin synthesis, suggesting that it can potentially improve innate immunity. While Vitamin D and TC combined increased cathelicidin production slightly, the effect was not statistically significant. Interestingly, neither vitamin D nor TC substantially affected TNF alpha production. The combination of vitamin D and TC, on the other hand, resulted in a considerable drop in TNF alpha levels, indicating a synergistic anti-inflammatory action. Conclusion: These findings suggest a possible avenue for exploiting vitamin D and TC's combined effects in enhancing immunological responses, particularly in cathelicidin production
К сожалению, текст статьи доступен только на Английском
Introduction. The innate immune system is the first component of the body's defense system that responds to an infection by a microorganism. One of the innate immune responses to infection is an increase in the production of CAP [1]. Research on cathelicidin (CAP) is essential, especially considering the recent rise in the incidence of infectious diseases and the rising concern over antibiotic resistance in microorganisms. CAP is a part of the innate immune system, especially humoral immunity, which plays a vital role in the homeostasis of immune systems [2]. Cathelicidins directly kill Gram-positive and Gram-negative bacteria and other pathogens. It can also increase phagocytosis by opsonizing bacteria and upregulating bacterial recognition receptors, degranulating neutrophils and mast cells, and upregulating inflammatory cytokines and cytokine receptors, which in turn increases intracellular toll-like receptor (TLR) signaling [3].
CAP synthesis can be stimulated naturally by the presence of an infection. On the other hand, the signaling mechanism of vitamin D through the vitamin D receptor (VDR) increases cathelicidin production, and therefore vitamin D induction increases cathelicidin synthesis [4]. It will be possible to design novel therapeutic strategies against a range of infectious disorders by comprehending how the vitamin D-cathelicidin axis functions in the human response to infection [5].
Proinflammatory cytokines such as TNF alpha are often expressed simultaneously at higher levels in granulocyte cells after increasing the level of CAP [6]. The body typically produces more proinflammatory cytokines in response to infection to bring various immune-competent cells to the infection site. However, there are situations where an overabundance of proinflammatory cytokines might worsen infections and inflammation.
On the other hand, plant extracts contain plenty of bioactive substances that may modulate the immunological response in the body. Many plants have been investigated for the content of anti-inflammatory substances such as polyphenols, flavonoids, and terpenoids [7]. Tinospora cordifolia (TC) is a plant widely used for traditional remedies in South and Southeast Asia. This plant has also been found to possess bioactive chemicals with anti-inflammatory properties. In silico analysis reveals that TC can potentially inhibit inflammatory activities by targeting and inhibiting the enzymatic activity of COX-2, suggesting a potential anti-inflammatory mechanism for TC [8]. TC has been shown to suppress TNF alpha and IL-1beta production in dendritic cells treated with LPS [9]. TC also inhibits inflammation in LPS-induced THP-1 cells by inactivating the NF-κB pathway, underscoring its potential as an anti-inflammatory agent targeting this essential signaling pathway [10].
CD66a, also known as Carcinoembryonic-antigen-related cell-adhesion molecule 1 (CEACAM1), is a marker that is expressed on the membrane of myeloid hematopoietic cells such as neutrophils, eosinophils, and basophils, which are collectively known as granulocyte cells [11]. Although neutrophils are the most dominant cells expressing CEACAM1 [12], this marker is also expressed by macrophages, B cells, T cells, NK cells, and platelets in mice [13].
The aim of the study. There has been limited research on combining vitamin D and plant extracts, particularly TC, in boosting CAP and regulating proinflammatory cytokines in granulocyte cells. Since CD66a subset cells, including neutrophils, express/produce CAP, this study focuses on monitoring the production of CAP and TNF alpha in CD66a subset cells after 28 days of administration of vitamin D, TC, and a combination of both and following treatment E. coli infection on day 29. The findings of this study are expected to determine the effect of combining vitamin D and TC treatment in increasing CAP production and controlling TNF alpha production after acute infection (6 hours after E. coli infection) as an effort to support the function of the innate immune system.
Materials and Methods
Ethical clearance
The Health Research Ethics Committee of the University of Muhammadiyah Malang granted ethical approval for the experimental animal treatment process with the number E.5a/254/KEPK-UMM/XII/2022.
Material preparations
Vitamin D (cholecalciferol) is a product of Blackmores VItamin D3 1000 IU in a liquid dosage form. Tinospora cordifolia (TC) herbal Simplicia was obtained and determined at the UPT Balai Materia Medika, Batu City, East Java, Indonesia. The simplicia was ground into powder and macerated for three days with 96% ethanol 1:3 (w/v). The macerate was filtered, and the filtrate was concentrated using a rotary evaporator until a stable weight. TC was then stored in the refrigerator at 4oC until it was utilized.
Animal and Treatment
The animals used in the present study were female BALB/c mice purchased from the Malang Wistar Farm in the Indonesian province of East Java's Dau District of Malang. A total of 25 normal female BALB/c mice weighing about 20-25 g and aged 6-8 weeks were kept in cages under regulated conditions. During the experiment, they were given free access to a standard pellet diet and water every day. Experimental mice were divided into five treatment groups:
- Normal Group: healthy mice were given a standard diet and daily administration of distilled water for 28 days without infection on the day 29th;
- Infected Group: healthy mice were given a standard diet and distilled water orally every day for 28 days and infected with E. coli on the 29th day;
- Vitamin D Group: mice were given an oral vitamin D dose of 100 micrograms/kg body weight every day for 28 days and infected with E. coli on the 29th day;
- Tinospora cordifolia Group: mice were administered orally with Tinospora cordifolia (TC) extract at a dose of 100 mg/kg body weight every day for 28 days and infected with E. coli on the 29th day;
- Combination group of vit D + TC + infected: mice were given oral vitamin D 100 micrograms/kg bw + TC extract 100 milligrams/kg bw daily for 28 days and were infected with E. coli on the 29th.
Sacrificing BALB/c mice model and cell isolation
The mice were dissected by neck dislocation on the 29th day of treatment, six hours after E. coli infection. The spleen was isolated. After that, Phosphate Buffer Solution (PBS) is used to cleanse the organs. Then, 1 mL of PBS was added to a Petri dish, and each organ was crushed clockwise. Then, the splenic suspension was centrifuged at 4°C for 5 minutes at 2500 rpm. 1 mL of PBS was added to the homogenate before centrifuging for 5 minutes at 2500 rpm and 4°C. The pellet was stained with the specific antibody once the supernatant was discarded.
Immunostaining and flow cytometry analysis
Pellets taken from the spleen were stained with FITC-conjugated anti-mouse CD66a for extracellular staining, and they were then incubated at 4 °C for 20 minutes in a darkened chamber. Samples were fixed with Cytofix/Cytoperm for intracellular staining, and they were then incubated at 4 °C for 20 minutes in a darkened chamber. Subsequently, the sample was mixed with 500 ml of intracellular staining and permeabilization wash solution and centrifuged at 2500 rpm for 5 minutes at 4°C. Anti-mouse TNF alpha conjugated with PE and anti-mouse CAP conjugated with Pe-Cy7 were used to stain spleen pellets. Every sample was incubated for 20 minutes at 4°C in a darkened chamber. Following incubation, flow cytometry (BD Biosciences FACS CantoII) was used to examine the samples. BD CellQuest ProTM was used to analyze the data and determine the relative sum of each parameter.
Statistical analysis
The data were presented as mean ± SE. The significant differences in the group were determined using one-way ANOVA, followed by an LSD post hoc test with p 0.05. The experiment was replicated three times.
Results and Discussion. This is an in vivo intervention study to assess the production of CAP and TNF alpha in the CD66a subset cells after treatment of vitamin D, Tinospora cordifolia extract (TC), or a combination of both. The main objective of this study was to determine the effectiveness of combining vitamin D and TC in increasing CAP production and controlling TNF alpha as proinflammatory cytokines, thus helping to increase the efficacy of CAP in reducing the risk of infection and preventing hyperinflammation.
The data shown in Fig 1 clearly illustrate how vitamin D supplementation affects CAP production in the mouse CD66a cell subgroup. Compared to the other treatments, vitamin D administration for 28 days seems to have a higher level of CAP production. Induction of E. coli infection in four groups other than the standard group appeared to elevate CAP production in CD66a cells, though not to the same level as the infection + vitamin D treatment. Interestingly, the infection + TC treatment showed that CAP production was identical to the healthy (non-infected) control. According to data analysis on the TC treatment, CAP production was lower than in the infection, vitamin D, and combination groups.
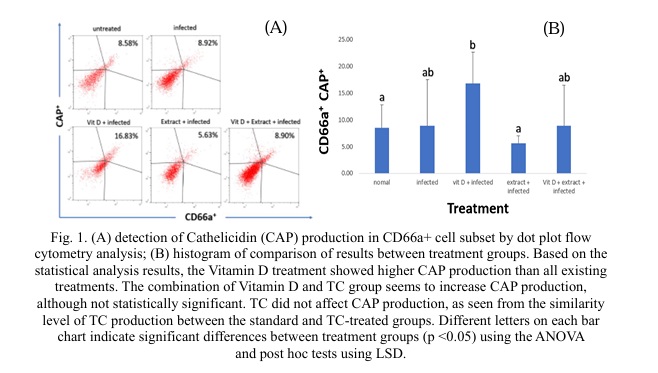
The administration of vitamin D demonstrates higher cathelicidin production compared to other treatments. Vitamin D [4] is one of the chemicals that effectively stimulates cathelicidin production. Vitamin D in the form of 1,25(OH)2D3, is a ligand of the vitamin D receptor protein (VDR) and activates a set of genes that control the innate and adaptive immune systems, including boosting cathelicidin production [14]. It is known that cathelicidin production is indeed derived from the activation of the vitamin D receptor (VDR) [15, 16] in various cell types, including macrophages and granulocytes in the blood [17]. Vitamin D influences the expression of cathelicidin by regulating the gene expression of cathelicidin hCAP/LL-37 and also impacts cytokine production while simultaneously regulating several vital physiological functions such as calcium and phosphate regulation, bone metabolism, including keratinocyte growth and differentiation, and bone formation [18].
Multiple pathways, including the MEK-ERK signaling pathway, regulate cathelicidin synthesis [19]. The MEK-ERK signaling pathway is involved in butyrate-mediated cathelicidin induction in colon epithelial cells. In addition, a recent study suggests that butyrate-induced expression of CRAMP depends on both ERK and p38 MAPK activities [20]. However, cathelicidin synthesis can vary depending on the type of cell. It is also synthesized under varying conditions. For instance, human cathelicidin antimicrobial peptide (CAMP)/LL-37 is regulated by vitamin D receptor (VDR)-dependent processes in various cell types. However, VDR-independent pathways involving endoplasmic reticulum (ER) stress signaling can also induce CAMP expression. Infections, wounds, UV radiation, and disruptions to the permeability barrier can all produce this stress signal [21].
TC did not affect CAP production, as seen from the similarity level of TC production between the standard and TC-treated groups. Nevertheless, some research results indicate that TC significantly enhances the innate immune system's function, albeit indirectly, by not increasing CAP production. TC contains the polysaccharide G1-4A, which can boost the innate immune system response by activating the TLR4 receptor pathway in macrophage cells [22]. In macrophage cells in vitro, water and methanol-extracted TC applications increase the immune response, characterized by elevated levels of proinflammatory cytokines such as TNF alpha, IFN gamma, and IL-1b [23]. Furthermore, in vivo studies have shown that TC extract administration can enhance the innate immune profile, particularly in infection prevention in experimental animals exposed to infections [24].
Fig. 2 shows that CAP production appears to be connected to TNF alpha production. In mice models, infection factors and vitamin D administration enhanced CAP and TNF alpha production, but TC administration decreased TNF alpha and CAP production. In this investigation, vitamin D and TC extract were administered to experimental mice to see if they may improve the immune system. Many vitamin D deficiencies are related to an increase in the severity of infectious diseases, and supplementing vitamin D in the diet can decrease the severity of infections [25].
Due to its ability to modulate the immune system, TC is categorized as an immunomodulatory plant [26], which means it can assist in regulating the immune system. However, there is some indication that TC may stimulate the immune system, which may be seen as immunosuppressive under certain situations. In RAW 264.7 cells, TC extract was observed to decrease TNF-alpha gene expression [27]. TC has been demonstrated to lower important immunological mediators of inflammation, such as TNF-alpha, in an autoimmune arthritis investigation [28]. TC chloroform extract was discovered to be supportive in reducing TNF-alpha production in THP-1 macrophages [10]. TC contains an extensive quantity of phenolic and flavonoid substances and has been shown to decrease the elevated production of TNF alpha in monocyte cells (THP-1) via the NF-kB pathway [7]. As a result, the combination of Vitamin D and TC is expected to increase CAP production while limiting inflammation.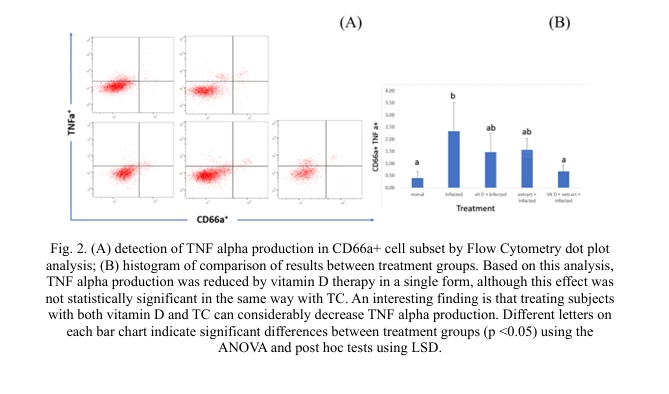
A marker known as CD66a, referred to as CEACAM1, is expressed on the membrane of neutrophils, eosinophils, and basophils, which are all recognized as granulocyte cells in the myeloid pathway of the hematopoietic origin [11]. In addition to serving as a marker, CEACAM1 regulates leukocyte functions such as angiogenesis, tumor growth, cell migration, and proliferation [29]. Although this marker is known to be expressed by a variety of leukocyte cells, including macrophages, B cells, T cells, NK cells, and platelets in mice, neutrophils are known to be the most dominant cells expressing CEACAM1 [12]. Granulocyte Monocyte colony-stimulating factor (GM-CSF) treatment can intensify CAECAM1 production in granulocyte cells [29].
In this study, E. coli was induced to observe the innate immune response, specifically in the CD66a leukocyte cell subset. E. coli's cell wall contains Lipopolysaccharide (LPS). LPS stimulates the immune system in intestinal epithelial cells by initially recognizing Toll-Like Receptor-4 (TLR4). TLR4 activation causes nuclear factor kappa B (NF-kB) and other transcription factors to be triggered, resulting in CAP production [30]. In addition to infection, administering certain natural chemicals, such as vitamin D and several plant bioactive components, can boost CAP production [31, 32]. On the other hand, giving several plant bioactive chemicals can also suppress pro-inflammatory cytokines like TNF alpha [33].
Conclusion. In conclusion, the findings from this study shed light on the responses of CD66a cells to different treatments. Vitamin D became a potent stimulator, significantly elevating cathelicidin (CAP) production. Tinospora cordifolia (TC) demonstrated the ability to suppress the synthesis of TNF alpha in these cells. Notably, vitamin D exhibited a more pronounced influence on the increased levels of CAP compared to other treatments. At the same time, TC showed potential in moderating TNF alpha production in CD66a cells. The combination of vitamin D and TC, on the other hand, resulted in a considerable drop in TNF alpha levels, indicating a synergistic anti-inflammatory action. In short, the findings of this study demonstrate the beneficial impacts of vitamin D on CAP formation as well as Tinospora cordifolia's ability to suppress TNF alpha synthesis in CD66a cells. Furthermore, it appears that the combination of vitamin D and TC has a synergistic anti-inflammatory impact, indicating a potential therapeutic strategy for modifying immune responses in the relevant conditions.
Financial support
Our Study is supported by the Lembaga Pengelola Dana Pendidikan (LPDP)/Indonesia Endowment Fund for Education of the Republic of Indonesia as the research funding provider. The number of the grant: 0001004/IPA/D/PDD-2020.





















Список литературы
Список использованной литературы появится позже.