Clinic-laboratory blood parameters and the contribution of renin-angiotensin system gene polymorphisms in pregnant women with preeclampsia
Aннотация
Background: Preeclampsia (PE) is a pregnancy-related condition linked to hypertension and proteinuria. It is a major cause of maternal and perinatal mortality. The aim of the study: The aim of this study was to investigate the effect of PE on blood parameters and to assess its diagnostic significance. It also investigated the association between renin-angiotensin system gene polymorphisms and susceptibility to PE. Materials and methods: We studied 40 pregnant women aged 25 to 40 years (10 with normal pregnancy, 10 with mild PE, 10 with moderate PE, and 10 with severe PE). We measured alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea, creatinine, and total protein levels in both normal and preeclamptic pregnancies. Furthermore, we genotyped 40 preeclamptic women and 60 normotensive pregnant controls for renin-angiotensin system gene polymorphisms. Multifactor dimensionality reduction (MDR) was carried out to assess the interactions among these polymorphisms. Results: The activity of ALT, AST, and ALP significantly increased in PE groups (mild, moderate, severe) compared to the control group (p=0.008-0.00001). Urea level significantly increased in severe PE (p=0.038). Creatinine levels increased significantly in moderate and severe PE (p=0.00004-0.00001), while total protein levels decreased significantly in all PE groups compared to the control group (p=0.001-0.00005). We also found that the T allele of the AGT (C521T; Thr174Met; rs4762) polymorphism was significantly associated with the risk of PE (p=0.005), and the TT genotype was also significant but not associated with increased risk of PE (p=0.034). The best MDR model was the four-factor model with a test balance accuracy of 0.546 and a maximum cross-validation consistency of 10/10 (p<0.0001). Conclusion: Preeclamptic pregnancies had elevated ALT, AST, ALP, serum urea, serum creatinine, and reduced total protein levels. In addition, the AGT (C521T; Thr174Met; rs4762) polymorphism was associated with PE risk. Furthermore, the four-factors MDR model was highly informative and associated with PE risk (p<0.0001)
К сожалению, текст статьи доступен только на Английском
Introduction. The problem of PE is one of the most important problems in modern obstetrics. The frequency of PE according to different estimates is from 2 to 10% of all pregnancies [1, 2, 3]. The special importance of PE is due to the high level of maternal and infant morbidity and mortality. PE accounts for 50000-60000 maternal deaths worldwide each year [4]. It can be the cause of premature detachment of the normally located placenta (PNRP), massive bleeding during and after delivery, and low birth weight babies [2, 5, 6]. Complications of PE also include placental insufficiency and delayed fetal development syndrome (FDDS) [7-10]. Long-term prognosis of women who have undergone PE is associated with an increased incidence of diabetes mellitus, obesity, coronary heart disease, strokes [8-11]. Children of women with PE are more likely to suffer from various metabolic, hormonal and cardiovascular diseases (CVD) [12].
Despite a long history of study, the etiology and pathogenesis of PE remain poorly understood. In this connection, biochemistry studies and molecular genetic studies are of great importance because they allow us to approach the elucidation of the causes of PE, to understand the molecular mechanisms of its development, and, in perspective, make it possible to perform early diagnosis, timely prevention and adequate treatment of this dangerous complication of pregnancy.
Of greatest importance in the outcome of pregnancy with PE is the development of hepatic and renal insufficiency [13, 14]. Enzymatic functions of the liver are impaired [15, 16]. As a criterion for assessing hepatocyte damage is the determination of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels [17]. In clinical practice simultaneous determination of AST and ALT activity in blood is widely used; it provides much more information on localization and depth of lesion, activity of pathological process; it allows to predict disease outcome [6]. Many clinical studies have found impaired liver function in pregnant women with PE and increased aminotransferase activity in the blood serum, which is an easily reproducible diagnostic test [18, 19]. In pregnant women, especially in the third trimester, there is also a physiological increase in the activity of alkaline phosphatase (ALP) enzyme, an additional source of ALP in this case is the placenta [20, 21].Very high numbers of ALP activity are observed in women with PE, which is a consequence of placental damage, while low activity of ALP in pregnant women indicates insufficient placental development. Furthermore, one of the well-known criteria for diagnosing the severity of PE is the determination of total serum protein. In addition, increased concentrations of nitrogenous products may reflect the occurrence and development of functional renal failure in pregnant women with PE. Therefore, it is important to investigate serum urea and creatinine levels for diagnostic purposes [14, 22]. Determination of serum urea levels, along with creatinine, is used to assess the excretory function of the kidneys.
Furthermore, the renin-angiotensin system (RAS) plays a role in the pathogenesis of PE [23]. The circulating RAS becomes dysfunctional in PE, and the RAS is proposed to play a role in its development [24]. Contrary to what would be expected in a state of primary volume reduction, the circulating RAS is suppressed in PE almost down to levels seen in non-pregnant women [25]. The compensatory alterations in the RAS contribute to the salt-water balance and sufficient placental perfusion for the mother and fetus [26]. Moreover, many genes are thought to be involved in the development of PE. These include genes encoding enzymes and receptors of the RAS [27], such as angiotensinogen (AGT (T704C; Met235Thr; rs699); AGT (C521T; Thr174Met; rs4762)) genes and angiotensin II gene receptors type 1 and type 2 (AGTR1 (A1166C; rs5186); AGTR2 (G1675A; rs1403543)). These polymorphisms were associated with elevated levels of AGT in serum in female patients with essential hypertension, resulting in increased SBP and DBP as well as abnormal uterine spiral artery remodeling, which is an early cause of PE development [28]. Furthermore, there is evidence that AGT (T704C), (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) polymorphisms may be implicated and play a significant role in pregnant women with PE [29]; however, the findings are still controversial. As a result, the purpose of this study is to determine the association between these polymorphisms and PE in Russian pregnant women from the Rostov-on-Don region.
The aim of the study. To study the effect of pregnancy complicated by PE on biochemical blood parameters in order to assess their differential diagnostic significance in relation to the severity of pathology and to investigate the relationship of renin-angiotensin system gene polymorphisms with susceptibility to PE.
Materials and Methods. The present study was conducted at Southern Federal University in collaboration with the Nauka Medical Center (Rostov-on-Don, Russia) during the period from 2019 to 2022. The study protocol was approved by the Southern Federal University Committee and written informed consent was obtained from each patient included in the study. A clinical and biochemical examination was made in 40 pregnant women aged 25 to 40 years. The first group consisted of healthy women with normal physiological pregnancies (10 samples). The second group included pregnant women with mild PE (10 samples), the third group included pregnant women with moderate PE (10 samples), and the fourth group included pregnant women with severe PE (10 samples). Moreover, the genetic analysis was performed on 100 pregnant women. The women were divided into two groups to study AGT genes SNP: women with PE (n=40) and women with normal pregnancy (n=60).
Obtaining biological material
Material for biochemical tests was collected on an empty stomach. We collected about 5 ml of blood (from both preeclamptic and normotensive pregnant women) in a plain vacutainer. We allowed a clot to form in a test tube for serum separation. After a 30-minute waiting time, the serum was separated by centrifugation at 3000 rpm for 10 minutes. The separated serum was used for the analysis of blood biochemical parameters, such as alanine aminotransferase activity, aspartate aminotransferase activities, alkaline phosphatase, urea, creatinine, and total protein content in the serum of pregnant women with normal pregnancy and preeclamptic pregnant women.
Enzyme activity measurement
Biochemical studies were performed using an automated rchitect C16000 analyzer (Abbott Diagnostics, Illinois, USA). Serum creatinine was estimated by the Jaffe method, serum urea by the urea nitrogen urease test, alkaline phosphatase activity by the para-nitrophenyl phosphate kinetic method, aspartate aminotransferase activity by an optimised enzymatic kinetic method, alanine aminotransferase activity by an optimised enzymatic kinetic method, and total protein concentration by the Biuret method. In clinical practice, the de Ritis coefficient (AST/ALT ratio), which is usually 0.91-1.75, is used for the differential diagnosis of liver and myocardial (muscle tissue) diseases. This coefficient is calculated on the basis of the organ specificity of the ALT (liver) and AST (heart) enzymes for the clinical differentiation of liver diseases, myocardial pathologies and destructive changes in skeletal muscle.
DNA isolation
Venous blood samples were collected in EDTA tubes and stored at −80◦ C. Genomic DNA was isolated from peripheral blood leukocytes using “PROBA-NK” extraction kit (“DNA-Technology”, Russia) according to the manufacturer’s protocol. Extracted DNA was measured for each sample and then stored at −20 °C.
Studied genetic variants and genotyping
We studied the following genes polymorphisms of angiotensinogen and angiotensin II type 1 and type 2 receptors: AGT (T704C; Met235Thr; rs699); AGT (C521T; Thr174Met; rs4762); AGTR1 (A1166C; rs5186); AGTR2 (G1675A; rs1403543) in Russian pregnant women from the Rostov-on-Don region. We chose these polymorphisms given their association with hypertension and because they affect all components of the RAAS. In addition, they have been widely studied in relation to hypertension in pregnancy, but with contradictory results. For all our knowledge, the reason for this inconsistency may be due to differences in geographic regions, ethnicity and sample size.
Genotyping was conducted with real-time polymerase chain reaction (RT- PCR) with Hypertension Susceptibility REAL-TIME PCR Genotyping Kit (“DNA Technology”, Russia).
For targeted groups of polymorphisms, fluorescent labels, and results were automatically registered with the DT-96 detecting amplifier (“DNA Technology”, Russia) following the manufacturer's instructions.
Statistical processing of results
Statistical analysis was run in Statistica 12. The Kolmogorov-Spearman and Shapiro-Wilk tests were used to test for normal distribution of quantitative measures in the groups being compared. If the data were not normally distributed, the non-parametric Mann-Whitney U test was used to compare two samples. If the distribution was normal, Student's t-test for small sample size was used. All p-values < 0.05 were considered significant. Hardy-Weinberg equilibrium was calculated using the X2 test. Estimates of the relative risk of PE are based on the calculation of odds ratios and P values. In addition, open-source multifactor dimensionality reduction analysis (MDR version 3.0.2, http://www.epistasis.org/) was performed to find gene interactions between the four polymorphisms.
Results. The results obtained indicate impaired functional activity of the liver and kidneys, the severity of which depends on the severity of the PE. In the PE group, the studied AGT (T704C), AGT (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) gene polymorphisms did not deviate from Hardy-Weinberg equilibrium. The functional state of the liver and kidneys was estimated on the basis of serum biochemical indices: the activity of ALT, AST, ALP, urea and creatinine content, and total protein level.
Table 1 shows that the level of serum ALT was significantly increased in PE of different severity (21.53±3.23; 23.72±3.87; 39.64±4.47) compared to control group (11.51±0.91) (p=0.008; 0.007; 0.00001). The level of AST was significantly increased in PE of different severity (14.70±0.95; 16.71±1.16; 28.62±3.11) compared to the control group (10.43±0.71) (p=0.002; 0.002; 0.00003). In addition, serum ALP levels were also significantly increased in PE of different severity (139.20±0.81; 168.70±6.39; 238.42±17.72) compared to the control group (130.40±2.12) (p=0.001; 0.00003; 0.00001).
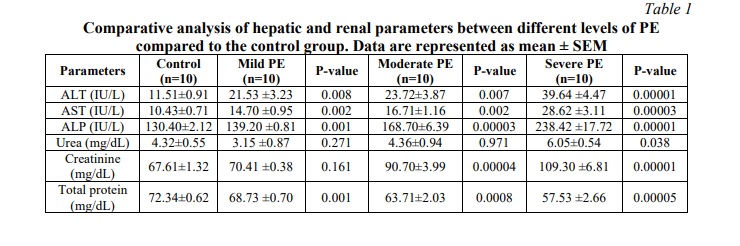
We also compared the level of serum urea in the control group with different severity of PE and we found that the serum urea level in pregnant women with mild PE insignificantly slightly decreased (3.15±0.87) as compared to the control group (4.32±0.55) (p=0.271), but varies within the control range in the moderate type of PE (4.36±0.94) (p=0.971). In the severe type of PE, the level of urea was significantly increased (6.05±0.54) (p=0.038) as compared to the control group. The level of serum creatinine was also insignificantly increased in mild PE (70.41±0.38) (p=0.161), whereas it was significantly increased in both moderate and severe PE (90.70±3.99; 109.30±6.81) compared with the control group (67.61±1.32) (p=0.00004; 0.00001). In addition, we measured total serum protein in different groups of PE and found that the total protein level was significantly reduced in mild, moderate and severe PE groups (68.73±0.70; 63.71±2.03; 57.53±2.66) compared to the control group (72.34±0.62) (p=0.001; 0.0008; 0.00005).
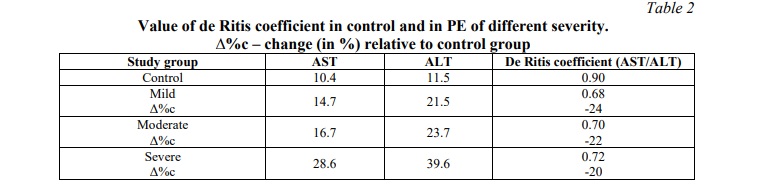
It should be noted that aminotransferases are characterized by organ specificity: ALT predominates in the liver, and AST – in the myocardium. To assess the ratio of AST and ALT activities in blood serum, the de Ritis coefficient was used, which may indicate organ damage to a certain extent.
As shown in table 2, the value of de Ritis coefficient in the control group with physiological pregnancy is 0.90, which corresponds to the literature data (0.8-1.0). In PE with different groups of severity, the value of de Ritis coefficient is 20-24% lower than normal. This indicates liver damage and is often associated with inflammatory diseases.
Allele and genotype frequencies in the PE and control groups
The allele and genotype frequencies of the AGT (T704C), AGT (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) polymorphisms in PE patients and healthy controls are reported in table 3. Significant differences were not observed among cases and controls in relation to genotype and allele frequencies for the AGT (T704C), AGTR1 (A1166C), and AGTR2 (G1675A( polymorphisms (p=0.363; 0.784; 0.880, and p=0.773; 0.578; 0.909, respectively).
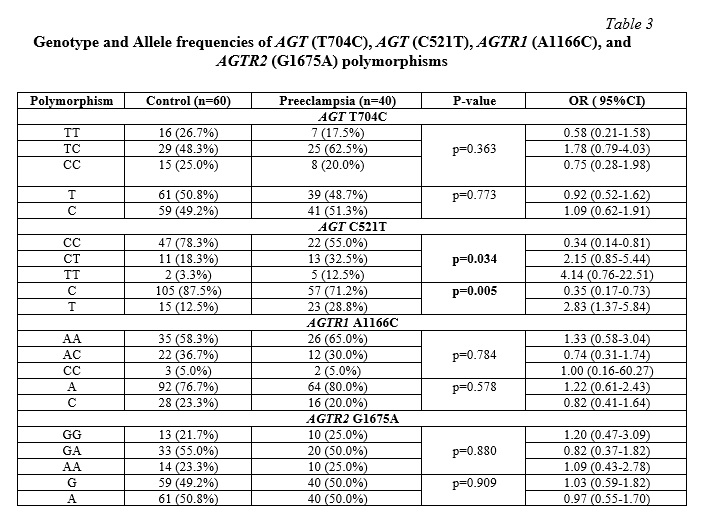
Significant differences in genotype and allele frequencies for the AGT (C521T) C>T polymorphism was only observed between cases and controls. The frequency of the TT homozygous genotype was higher in the PE group (12.5%) than in the controls (3.3%), and this difference was statistically significant (p=0.034). The T allele was also found to be significantly different in pre-eclamptic women compared to normotensive pregnant women (p=0.005), with a difference in allele frequencies in PE patients (28.8%) compared to controls (12.5%).
Multiple-locus interactions for four renin-angiotensin system gene polymorphisms
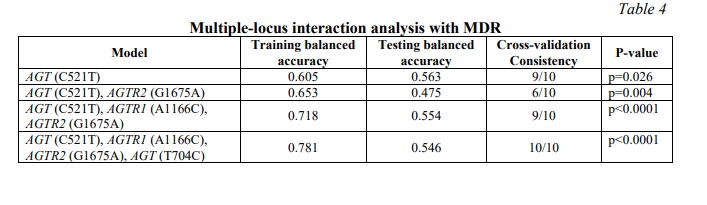
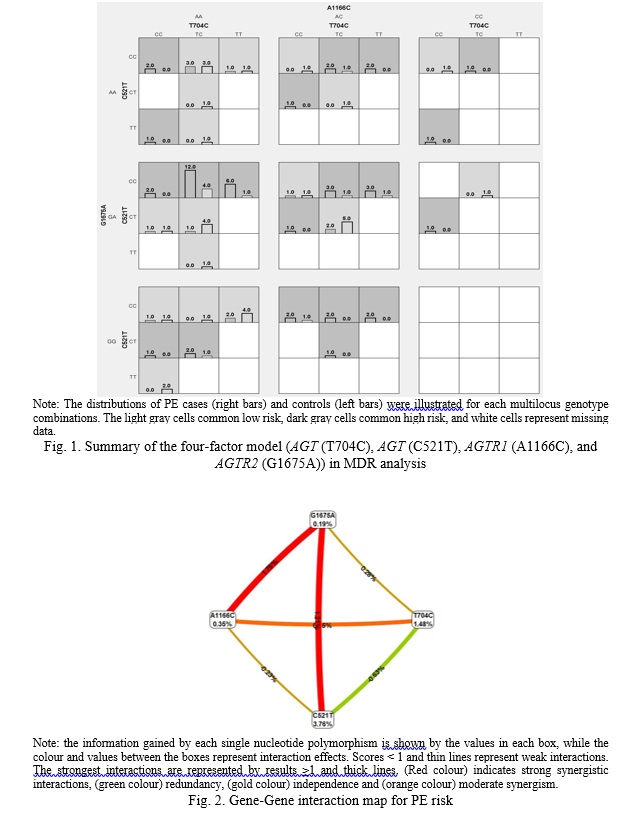
The possible interactions between polymorphisms of AGT (T704C), AGT (C521T), AGTR1 (A1166C) and AGTR2 (G1675A) genes in study groups were investigated, data are shown in table 4. According to the MDR analysis, the most informative MDR model was the four-factor model for the AGT (T704C), AGT (C521T), AGTR1 (A1166C) and AGTR2 (G1675A) polymorphisms (Figure 1). The model has a test balance accuracy of 0.546 and a training balance accuracy of 0.781 with a maximum cross-validation consistency of 10/10 (p<0.0001). Other possible interactions between polymorphisms are shown in (Figure 2). The analysis suggests that AGT (C521T) was the best one-factor model with a cross-validation consistency of 9/10 and balanced testing accuracy of 0.563 (p=0.026). The AGT (C521T), AGTR2 (G1675A) was the best two-factor model with a cross-validation consistency of 6/10 and testing balanced accuracy of 0.475 (p=0.004). While the best three-factor model was the combination of AGT (C521T), AGTR1 (A1166C) and AGTR2 (G1675A) with a cross-validation consistency of 9/10 and testing balanced accuracy of 0.554 (p<0.0001).
Discussion. Carrying out a certain complex of examinations in a pregnant woman makes it possible to predict the course of pregnancy and childbirth, possible complications, and, therefore, to make timely correction aimed at reducing the risk of diseases of the mother and fetus [30]. Biochemical blood tests in pregnancy can detect disorders of internal organs at a stage when there are no clinical indications yet. All this allows the diagnosis of pathology at an early stage.
Characteristic signs of PE, along with hypertension and proteinuria, are structural and functional disorders of the uteroplacental complex, which is accompanied by defects and ischemia of the placenta, abnormal remodeling of the spiral arteries, angiogenic imbalance, activation of inflammation, endothelial dysfunction and oxidative stress in the mother-placenta-fetus system [31]. All these disorders in PE in pregnant women contribute to the development of multiple organ failure with the greatest disorders in the tissues of the liver, kidneys, and brain [32].
The development of hepatic and renal insufficiency during pregnancy with PE is of paramount importance [33]. The progressive growth of the gestational process results in an increased load on the liver, placing it under functional stress. During pregnancy, the liver undergoes a depletion of its reserve capacity, making it susceptible to several vulnerabilities. Hepatic enzymatic capacity is compromised in individuals with PE [34]. In this particular scenario, the presence of clinical symptoms indicative of liver injury is often absent, yet alterations in hepatobiliary parameters can be detected in the preclinical stage [35]. The study [36] showed that elevated levels of ALT in the early stages of pregnancy correlate with an increased likelihood of developing preeclampsia in later stages of pregnancy. This finding highlights the importance of the biochemical test as a predictive tool. Typically, the assessment of hepatocyte damage involves the assessment of enzymatic activity levels of AST and ALT.
ALT and AST are key enzymes in amino acid metabolism that catalyze the reversible process of transamination to form glutamate and ketoacids. Aminotransferases are widely distributed in various tissues, but ALT is predominantly localized in the liver, and AST in the heart muscle [37]. ALT is also found in muscle, adipose tissue, intestines, colon, prostate and brain; however, ALT level in these organs are significantly lower than in the liver. In addition, ALT activity in the liver is approximately 3000 times higher than that in the serum.
Thus, in the case of hepatocellular damage, which develops in PE due to the activation of free radical oxidation and the formation of highly reactive oxygen species and lipid peroxidation products [31], the structure is disrupted and the permeability of hepatocyte membranes increases. As a result, ALT is released from damaged liver cells and causes a significant increase in serum ALT activity.
Our study revealed an increase in aspartate aminotransferase and alanine aminotransferase activity in pregnant women with PE compared with the control group. Increased enzymatic activity was observed in mild, moderate, and severe PE. This result indicates a direct dependence of hyperenzymemia in the serum on the degree of damage to hepatocytes. In addition, alkaline phosphatase activity in blood serum increases in all groups. In the second trimester of pregnancy, fetal placental alkaline phosphatase begins to enter the maternal bloodstream. The woman's alkaline phosphatase level increases and reaches its maximum in the third trimester.
Furthermore, it is known that in the development of PE an important link in pathogenesis is a disturbance of functional activity of kidneys that are affected before other organs, leading to nephropathy development [13, 38, 39]. Our study also looked at serum levels of the nitrogenous products of protein metabolism, urea and creatinine. Compared to the control group, urea level fluctuations in pregnant women with mild and moderate PE were within normal limits. With a severe group of PE, serum urea concentration significantly increased compared to the control group. Serum creatinine concentration remained within the control limits in the group with mild PE and significantly increased in moderate and severe groups of PE as compared to the control group. The observed increase in creatinine and urea levels can be interpreted as an indication of increased cell membrane permeability to small molecules. Conversely, an increased concentration of nitrogenous compounds may indicate the presence and progression of latent renal dysfunction.
We also observed hypoproteinemia in blood serum in PE with different degrees of severity. Changes in the content of total protein in PE reflect either a decrease in its synthesis, usually by the liver, or increased loss in the kidneys or with intestinal fluid, or its increased consumption in pathological processes, including disseminated intravascular coagulation [40, 41].
Our study found that the determination of serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, urea, creatinine and total protein activity is a diagnostic criterion of the severity of PE. The most informative indicators associated with the severity of the pathological process and having diagnostic value are the serum aminotransferase and alkaline phosphatase activity levels in pregnant women with various degrees of PE.
It should be noted that the disturbance of the metabolic status in PE in pregnant women is closely related to the imbalance of the amino acid spectrum of blood serum and amniotic fluid, which may indicate a violation of amino acid and protein metabolism. Amino acids play a crucial role in protein biosynthesis and also serve as precursors for various bioactive compounds, such as neurotransmitters, nitric oxide, and antioxidants. Additionally, they are involved in energy metabolism, regulate immune responses, induce hormone synthesis, and activate proliferative processes within the fetoplacental complex [42].
A study [43] showed that the levels of glutamate, serine, glycine and phenylalanine were increased in the maternal serum of patients with preeclampsia. Glutamate increased the risk of preeclampsia by a factor of 5.5, while low levels of methionine may increase the risk of preeclampsia by a factor of 3.6. Changes in the metabolic status of amino acids have been found in the umbilical cord blood of newborns of women with preeclampsia compared with normal pregnancies [44]. The work [45] notes a modification of the amino acid spectrum of amniotic fluid in pregnant women with PE, with the most significant imbalance in the second trimester of pregnancy, reflecting a violation of homeostasis in the mother-placenta-fetus system.
It is thought that the activation of the RAS, which is the body's natural response to low blood pressure and low blood volume, results in increased vascular resistance and increased blood pressure, which can lead to PE [46]. Additionally, the RAS has been linked to the development of abnormal placental vascularization, which is thought to be a major factor in the development of PE [47]. Studies have found that increased levels of the RAS components, angiotensinogen, angiotensin converting enzyme, and angiotensin II, have been linked to an increased risk of PE [48, 49]. Additionally, increased levels of angiotensin II have been linked to an increased severity of PE [50]. It is thought that the RAS increases the production of pro-inflammatory cytokines, which can lead to increased vascular permeability and decreased blood flow to the placenta, leading to placental ischemia and the development of PE [51].
The mechanistic explanation for the involvement of AGT (T704C) in the development of PE has previously been proposed to occur via a localized increase in Ang II levels, leading to aberrant physiological remodeling of the uterine spiral arteries [52]. Women with PE who had the T704C C/C or 235 T/C genotypes exhibited elevated levels of plasma angiotensinogen AGT in comparison to those with the T704C T/T genotype. This observation was accompanied by a concomitant rise in angiotensin II (Ang II) production, hence leading to heightened vascular tone and vascular hypertrophy [53]. The presence of the AGT (T704C) minor allele, in conjunction with the AGT promoter –6G>A, was shown to be correlated with increased AGT expression [54]. Furthermore, there was a correlation between placental abruption resulting from impaired spiral artery remodeling and the presence of the AGT (T704C) minor allele in 63% of women diagnosed with PE, as well as in 60% of cases with intrauterine growth restriction, as compared to pregnancies that progressed normally. Conversely, the minor allele frequency (MAF) of AGT (C521T) exhibits lesser prevalence in Tunisians, as well as in European, Asian, and African American groups [55]. However, it has been shown that the MAF of AGT (C521T) is less frequent in instances of PE compared to pregnant women in the control group [56]. The AGTR1 and AGTR2 genes provide instructions for making the type 1 and type 2 angiotensin cell receptors that respond to angiotensin II, respectively. The expression of the AGTR1 gene is boosted when the adenine (A) at position 1166 in the gene's regulatory region is switched for the cytosine (C). The subsequent events trigger the amplification process. The AGTR1 1166A allele, when translated into mRNA, contains non-coding regions that interact with microRNA miR155 and thereby inhibit translation, resulting in a decrease in protein synthesis during receptor protein synthesis. The AGTR1 1166C allele enhances protein synthesis and alters the functional activity of the receptors because microRNAs cannot bind to it [57]. The interaction of angiotensin II with type 2 receptors leads to a reduction in blood pressure, in contrast to the effects of AT1 receptors on the cardiovascular system. The AGTR2 1675G allele is associated with transcriptional activation and therefore determines how many angiotensin II type 2 receptors are expressed on the cell surface. The control of gene expression is adversely affected by the G1675A nucleotide substitution in the regulatory domain of the gene. Carriers of this low-functioning polymorphism have fewer type 2 receptors and a reduced ability for these receptors to perform their normal functions (including NO generation and arterial dilation), putting them at higher risk of hypertension.
According to several studies, the renin-angiotensin system (RAS) has been implicated in the pathogenesis of PE [58]. As for AGT (T704C), a meta-analysis study found that the AGT (T704C) polymorphism is associated with PE [28]. Another meta-analysis [59] showed that the TT genotype elevated PE risk when compared to the TT genotype (OR=1.61, 95%CI=1.22-2.14, p=14.001). On the other hand, a study by [60] confirmed that no differences were observed between the control and PE groups for AGT (T704C), which is consistent with our finding for this polymorphism. Regarding AGT (C521T), a meta-analysis study by [59] showed that no association was found for AGT (C521T) with PE, which is in contrast to the findings of [28]. Similarly, another study by [61] showed that the AGT (C521T) variant contributes to an increased risk of developing PE, which is also in agreement with our results for AGT (C521T). Furthermore, as for the AGTR1 (A1166C) polymorphism, a meta-analysis study [28] found that in the Asian population there was an association between the C allele and AC/CC genotypes and the risk of hypertensive disorders in pregnancy. On the other hand, no association was found between the AGTR1 (A1166C) gene polymorphism and PE in women from Iranian, Afro-Caribbean and Caucasian populations. Similar results have been published in meta-analyses that did not find the A1166C polymorphism to be a predisposing locus for the development of PE. As for AGTR2 (G1675), a study by [62] showed that the presence in the genotype of the G1675A allele in the angiotensin II type 2 receptor gene is a risk factor for the development of chronic arterial hypertension, whereas this is contrary to our finding for the AGTR2 (G1675) polymorphism.
In our study, the associations of polymorphisms in AGT (T704C), AGT (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) with PE were analyzed as well as the gene-gene interactions using MDR. We found that the T allele of the AGT (C521T) polymorphism was significantly associated with the risk of PE (p=0.005), while, TT genotype was also significant but not associated with increased risk of PE (p=0.034, (OR= 4.14 95% CI (0.76-22.51)). No significant differences in genotype and allele frequencies for the AGT (T704C), AGTR1 (A1166C) and AGTR2 (G1675A) polymorphisms were observed between cases and controls (p=0.363; 0.784; 0.880; and p=0.773; 0.578; 0.909, respectively). In other words, the results of the study show that the AGT (T704C), AGTR1 (A1166C), and AGTR2 (G1675A) polymorphisms have no effect on the pathophysiology of PE in Rostov-on-Don pregnant women, while the minor allele of the C521T polymorphism was significantly different between pre-eclamptic women and the control group. Thus, this allele was associated with a high risk of developing PE (OR=2.83, 95%CI=1.37-5.84, p=0.005).
At the same time, the analysis of intergenic interactions showed that the four-locus model of AGT (T704C), AGT (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) gene interaction is associated with the risk of PE development (OR=13.01, 95%CI=4.69-35.99, p<0.0001). The model has a testing balance accuracy of 0.546 and a training balance accuracy of 0.781 with a maximum cross-validation consistency of 10/10. These four polymorphisms combination was more predictive of PE than any three of the polymorphisms alone. This supports the theory that PE develops through genetic interference.
The current study has certain limitations. The sample size was rather small. Furthermore, we only studied a small number of polymorphisms. Nonetheless, more studies are required to understand the etiology of PE.
Conclusion. Based on our results, the most informative and diagnostically valuable in the characteristic of various degrees of PE are enzymatic tests: ALT > AST > ALP, the dynamics of nitrogenous catabolism products, urea and creatinine, are less informative; the test for determining total protein has the lowest diagnostic value and sensitivity. Therefore, we conclude that elevated serum ALT, AST, ALP, creatinine and urea levels are better diagnostic and predictive markers for differential assessment of the severity of РЕ. Furthermore, to the best of our knowledge, this is the first study to investigate the association between polymorphisms of angiotensinogen, angiotensin II gene receptors type 1 and type 2 and PE susceptibility in Russian pregnant women from the Rostov-on-Don region. We found an association between the angiotensinogen AGT (C521T) polymorphism and the risk of PE, suggesting that this polymorphism contributes to an increased risk of developing PE in pregnant women from Rostov-on-Don, whereas no significant differences in genotype and allele frequencies were found between cases and controls for the AGT (T704C), AGTR1 (A1166C) and AGTR2 (G1675A) polymorphisms, indicating that they are not genetic risk factors for PE in this population of Russian pregnant women from the Rostov-on-Don region. Moreover, the effect of gene-gene interactions on PE showed that the interaction of genes AGT (T704C), AGT (C521T), AGTR1 (A1166C), and AGTR2 (G1675A) is associated with the risk of PE; at the same time, this confirms the fact that the development of PE is not determined by a single gene, but depends on the functioning of a complex of genetic factors, and this may guide further studies to confirm our findings with different ethnic groups and a larger sample size.
Financial support
This study was funded by the Ministry of Science and Higher Education of the Russian Federation № FENW-2023-0018.





















Список литературы
Список использованной литературы появится позже.