Chemical composition and antimicrobial activities of Boesenbergia xiphostachya
Aннотация
Background:Boesenbergia xiphostachya is a rare species, recently discovered in Vietnam. Information about the chemical compositions and biological properties of distinct parts of this plant is still limited to date. The aim of the study: This study is the first to investigate the chemical composition and antimicrobial properties of acetone extracts and essential oil obtained from samples of B. xiphostachya collected in Vietnam. Materials and methods: Gas chromatography-mass spectrometry was used to identify the phytochemical components, while the antimicrobial properties of the essential oil and acetone extracts from the rhizome and aerial parts were determined using a disk diffusion assay. Results: A total of thirty compounds have been identified in the essential oil, in which α-fenchene (41.70%), cyclofenchene (14.75%), camphene (13.47%), and caryophyllene (6.78%) are the major compounds. In addition, the acetone extracts obtained from the aerial part and rhizome comprise twenty-two and twenty-seven compounds, respectively. The major constituents are mainly composed of n-hexadecanoic acid (21.32%), 2-pentanone, 4-hydroxy-4-methyl (19.19%), and phytol (7.78%), while the rhizome extract contains a majority of n-hexadecanoic acid (28.34%), benzoic acid, 2-amino-3-methoxy-, methyl ester (12.25%), and cinnamic acid (11.42%). Moreover, the essential oil and the acetone extracts from B. xiphostachya also possessed antimicrobial properties against a wide spectrum of microorganisms. Conclusion: The results obtained show that B. xiphostachya has great potential for use in biomedicine and related fields
Ключевые слова: acetone extract, antimicrobial activity, Boesenbergia xiphostachya, chemical composition, essential oil
К сожалению, текст статьи доступен только на Английском
Introduction. Boesenbergia Kuntze is a genus belonging to Zingiberaceae family with about 82 species distributed in south-western India, China, and Southeast Asia. Notably, six species have been recorded in the flora of Vietnam [1, 2]. The Zingiberaceae family is known for its medicinal properties and is used in traditional medicine to improve health [3, 4, 5].
Similarly, the extracts obtained from some species of Boesenbergia are broadly used as functional foods and traditional medicine in some Asian countries [1, 6, 7]. Furthermore, previous studies have reported on the biological activities and phytochemical profiles of Boesenbergia members. For instance, the methanol extract from B. rotunda had an inhibitory effect on 24 Leptospiral strains, reduce ulcer inflammation, decrease nasopharyngeal carcinoma HK1 cells viability, PANC-1 human pancreatic cancer cells[8-11], while the essential oil was found to be effective against Escherichia coli, Pseudomonas aeruginosa, B. cereus, S. aureus. It also suppresses biofilm formation of methicillin-resistant Staphylococcus aureus (MRSA) [12, 13]. The ethanolic extract of B. rotunda showed promise against SARS-CoV-2 infection in hamsters, along with antimicrobial, anti-inflammatory, antioxidant, wound healing activities [14, 15, 16], and inhibits Candida albicans biofilm formation [17]. Moreover, the bioactive compounds of Boesenbergia species have been investigated for their various bioactivities. For example, panduratin A and hydroxypanduratin A inhibit the production of TNF-α, nitric oxide and PGE2, and have anti-SARS-CoV-2 and periodontal treatment properties [18-21]. Similarly, panduratin A has been reported as a preventive agent against the growth of certain cancer cell lines [22, 23]. Additionally, longiferone B and C isolated from B. longiflora rhizome exhibit anti-inflammatory activity [24].
Boesenbergia xiphostachya (Gagnep.) Loes, a species belonging to Zingiberaceae, is mostly found in Thailand and Indochina [25]. The chemical compositions and biological effects of this species have been investigated by only two prior studies so far [26, 27]. For example, the ethanolic and dichloromethane extracts from the rhizome were found to have free radical scavenging activity [26], whereas the hexane extract exhibited only minor cytotoxicity activity on many cancer cell lines as well as slightly inhibited some Gram positive and negative bacterial strains [27]. This study, thus, provides the phytochemical and antimicrobial properties of the essential oil and the acetone extracts obtained from B. xiphostachya for the first time.
The aim of the study. This study is the first to investigate the chemical composition and antimicrobial properties of acetone extracts and essential oil obtained from B. xiphostachya.
Materials and methods
Plant materials
Plant samples of B. xiphostachya were collected from Gia Canh commune, Dinh Quan district, Dong Nai province, Vietnam. The vouchered specimens (DQ04082022) were deposited in the Herbarium of the Faculty of Natural Resources and Environment, Vietnam National University of Forestry-Dongnai Campus.
Microbial strains
Eleven bacterial strains and three yeast strains were used in this study, including Escherichia coli ATCC 25922, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 29213, Staphylococcus aureus ATCC 25923, Enterobacter hormaechei ATCC 700323, Salmonella typhimurium ATCC 13311, Klebsiella pneumoniae ATCC 700603, Klebsiella pneumoniae ATCC 13883, Shigella flexneri ATCC 9199, Staphylococcus saprophyticus BAA750, Vibrio parahaemolyticus ATCC 17802, Candida albicans ATCC 26790, Candida krusei ATCC 6258, Candida tropicalis.
Distillation of the essential oil
Five hundred grams of fresh rhizomes were washed, sliced into small pieces and submerged in 2.0 liters of distilled water. Steam distillation was carried out for 3 hours at normal pressure in a Clevenger apparatus. About 0.3 mL of essential oil was collected and stored in a dark bottle at 4℃ until analyzed.
Acetone extract preparation
Samples of rhizome and aerial part were dried at 50℃ then grinded into powder of which 100 g were soaked in 500 mL of 99% acetone solution (Thermo Fisher Scientific, USA) for 48 hours. The extract was collected by filtration and the filtered residue was re-extracted two more times. The pooled filtered extract was subjected to solvent removal under vacuum condition at 45℃.
Gas chromatography-mass spectrometry (GC/MS) analysis
The Thermo Scientific™ ISQ™ 7000 GC-MS system (Thermo Fisher Scientific, USA) was used to determine the composition of the sample as previously described by Nguyen et al. [28]. A gas chromatograph GC TRACE 1310 coupled with a single quadrupole mass spectrometer ISQ 7000 (Thermo Fisher Scientific, USA) was used to determine the chemical composition of the sample. Agilent GC column DB-5MS (30 m, 0.25 mm, 0.25 µm) was used as the stationary phase. The Helium carrier gas flow rate was set to 1.2 mL/min. The sample (1.0 µL) was injected into the GC system with the injector temperature maintained at 250 °C. The system was operated in split mode with a split flow of 36 mL/min and a split ratio of 30:1. The splitless time was set to 1 min. The oven programme was set to 80°C for five minutes; 20℃/min to 280℃; hold for 10 minutes; 20℃/min to 300℃; hold for 3 minutes. The transfer line temperature was 280℃, the ion source temperature was 250℃, and the ionization energy was 70 eV. The scan range was 29-650 m/z, dwell time was 0.2 seconds. The acquired mass spectral data were then compared with the NIST 2017 library to determine the exact chemical composition.
Determination of antimicrobial activity
Disk diffusion method was employed for the antimicrobial activity test following CLSI standards and guidelines [29]. Extract solution was prepared with various concentrations by dissolving into 20% DMSO solution. Gentamicin and ketoconazole disks (10 µg) were used as the positive controls for antibacterial tests and antifungal tests, respectively while 20% DMSO solution was used as the negative control. The experiment was performed three times and the data were analysed using Statgraphics Centurion XV software (ANOVA, α = 0.05).
Results and Discussion
Chemical composition of essential oil from B. xiphostachya rhizome
The essential oil derived from the rhizome of B. xiphostachya exhibits a phytochemical profile comprising 30 distinct compounds. α-fenchene (41.70%) was the most abudant component in the studied oil, followed by other notable compounds, including cyclofenchene (14.75%), camphene (13.47%), caryophyllene (6.78%), eucalyptol (4.83%), endo-borneol (2.30%), and bornyl acetate (3.30). Each of the remaining compounds represents less than 2% of the total composition (see Table 1).
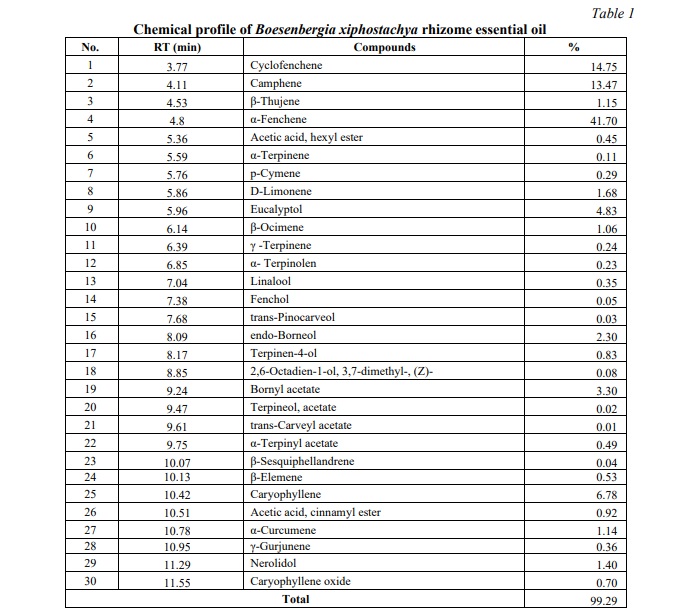
The identified compounds show a high similarity to those found in the essential oil of B. longiflora, including camphene, α-terpinene, p-cymene, β-ocimene, p-cymenene, and borneol [30]. Notably, the main component, α-fenchene, is also present in B. rotunda rhizome, albeit in substantially smaller quantities (2.01%). Despite these similarities, significant differences exist in the phytochemical profiles of the essential oils derived from these Boesenbergia species, both in terms of the compounds present and their respective compositions [12, 31].
The chemical composition of the acetone extracts from the aerial parts and rhizome of B. xiphostachya
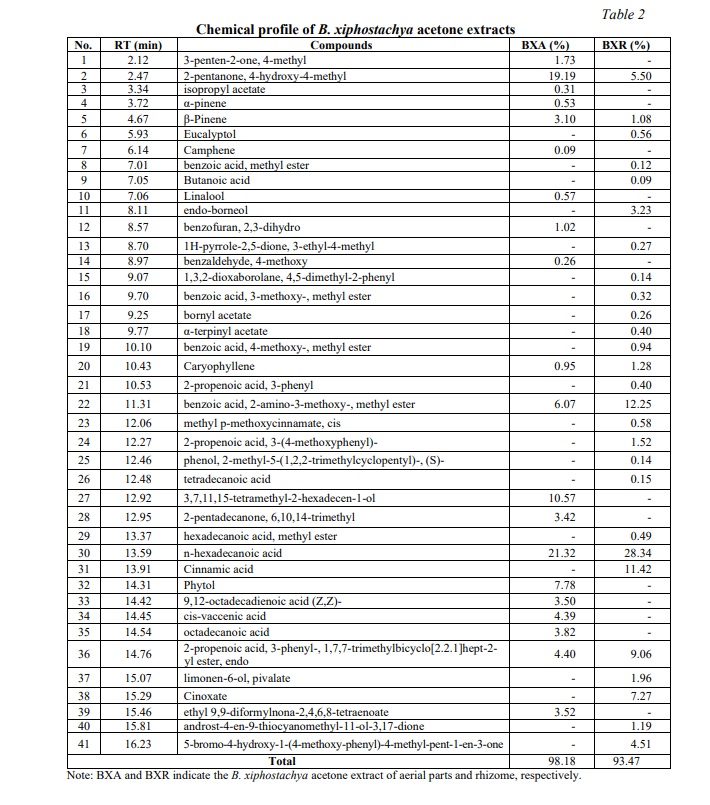
Table 2 presents the chemical composition of the acetone extracts derived from the aerial parts and rhizome of B. xiphostachya with a total of 21 and 27 compounds, respectively. The extract from the aerial parts primarily consists of n-hexadecanoic acid (21.32%), 2-pentanone, 4-hydroxy-4-methyl (19.19%), phytol (7.78%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (7.63%), and benzoic acid, 2-amino-3-methoxy-, methyl ester (6.07%). Meanwhile, the rhizome extract contains n-hexadecanoic acid (28,34%); benzoic acid, 2-amino-3-methoxy-, methyl ester (12,25%); cinnamic acid (11,42%); 2-propenoic acid, 3-phenyl-, 1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester, endo (9,06%); and cinoxate (7,27%) as the major compounds. The presence of identified compounds in the rhizome acetone extract differs significantly from that in the rhizome hexane extract. The main compound recorded in the latter was 3,6-dimethoxy-2-ethylbenzaldehyde (38.43%) [27]. Nevertheless, the high concentration of n-hexadecanoic acid in B. xiphostachya extracts suggests the presence of beneficial biological activities, such as antibacterial, antioxidant and anti-inflammatory properties, as reported in previous studies [32, 33]
Antimicrobial activity of essential oil and acetone extracts from B. xiphostachya aerial part and rhizome
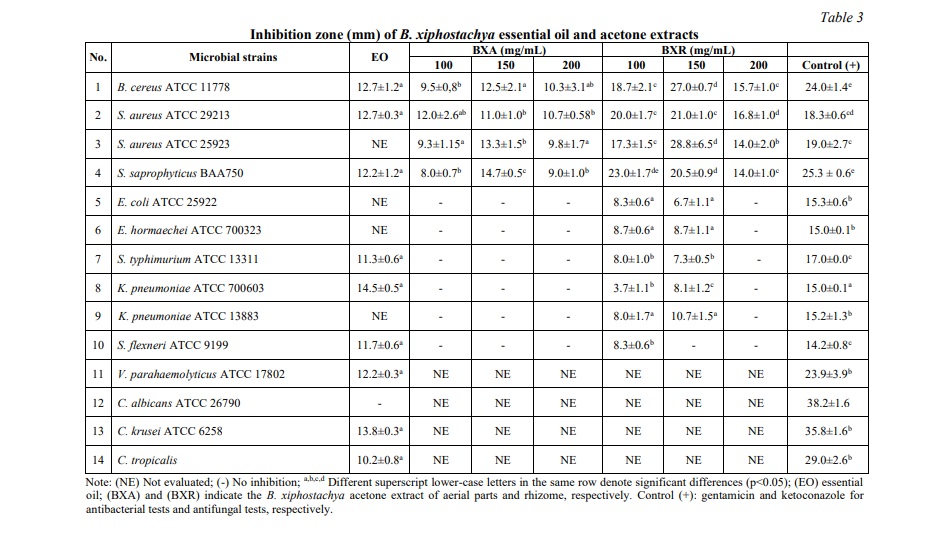
As this is the first study of B. xiphostachya essential oil, its results show promising antimicrobial results against seven strains of bacteria, including both Gram-negative and Gram-positive bacteria, as well as two strains of Candida. Specifically, the rhizome essential oil showed antimicrobial activity against B. cereus, S. aureus ATCC 29213, S. typhimurium, K. pneumonia ATCC 700603, S. flexneri, S. saprophyticus, V. parahaemolyticus, C. krusei, and C. tropicalis with inhibition zone diameters ranged from 11.3±0.6 mm to 14.5±0.5 mm (Table 3). The inhibition zones achieved in the antibacterial activity were more than 50% of the gentamicin inhibition zone. Especially, the inhibition zone against K. pneumoniae ATCC 700603 was comparable to the gentamicin control. In the case of Candida spp., the antifungal activity resulted in an inhibition zone exceeding 35% of that produced by ketoconazole. Notably, these inhibition zones are comparable to the results achieved with the essential oil of B. rotunda, which exhibited antibacterial activity against B. cereus and S. aureus with inhibition zones ranging from 13.0±4.58 mm to 16.0±3.0 mm [12]. The presence of the prominent compounds cyclofenchene, camphene, caryophyllene and eucalyptol in B. xiphostachya essential oil plays a pivotal role in its antimicrobial activity, as extensive research on plant essential oils has consistently reported the presence of these compounds and their antibacterial and antifungal effects [34-38]. In addition, while the biological activity of most terpenes and their derivatives against microbes is well-established, the speculative contribution of α-fenchene, a major component in the B. xiphostachya essential oil to the observed antimicrobial activity against the tested microbial strains remains to be elucidated [39].
Interestingly, the acetone extract from the B. xiphostachya aerial part exhibited antibacterial activity exclusively against four tested Gram-positive strains. This selective efficacy exhibited a minor variation with the concentration of the extract, which ranged from 100 mg/mL to 200 mg/mL (Table 3). Conversely, the rhizome extract exhibited stronger activity against all ten of the tested strains, with the exception of V. parahaemolyticus. Furthermore, it was observed that a high concentration of the extract, specifically at 200 mg/mL, resulted in a reduction of antibacterial activity against Gram-positive strains and a complete loss of activity against Gram-negative strains. Similar activity to the positive control was observed for the rhizome extract against the tested bacterial strains, such as S. aureus ATCC 25923 and S. saprophyticus. In the case of B. cereus, stronger activity was recorded at a concentration of 150 mg/mL. With the exception of antibacterial activity against K. pneumoniae ATCC 700603, which showed about 25% of the activity of gentamicin, antibacterial activity against other tested Gram-negative bacteria exhibited over 45% of the activity of gentamicin (Table 3). Compared to the hexane extract from B. xiphostachya, which exhibited weak inhibitory activity against B. cereus, E. faecium, E. coli, P. aeruginosa, K. pneumoniae, and C. albicans, the acetone extract in this study displayed superior antibacterial activity [27]. Acetone is an excellent choice for studying plant extracts for several reasons. Its intermediate polarity enables it to efficiently extract a wide range of organic compounds, making it ideal for isolating solutes or analytes with a similar polarity, and for collecting suitable chemical compounds. This could potentially be attributed to the presence of key compounds such as n-hexadecanoic acid and methyl 2-amino-3-methoxybenzoate (also known as benzoic acid, 2-amino-3-methoxy-, methyl ester) in the acetone extracts, and notably, the presence of cinnamic acid in the rhizome extract (Table 2). In addition, n-hexadecanoic acid has been identified to possess antibacterial properties against S. aureus, B. subtilis, E. coli, and K. pneumoniae [32]. Concurrently, benzoate derivatives and cinnamic acid have been verified to exhibit antimicrobial properties against a range of organisms, including but not limited to S. aureus, Pseudomonas aeruginosa, E. coli, Aspergillus spp., and Candida spp. [40-43].
Conclusion. This research presents the first exploration into the chemical profiles and antim icrobial properties of the essential oil and acetone extracts from B. xiphostachya, a newly discovered species in Vietnam. Identifying α-fenchene as the primary component in the essential oil and n-hexadecanoic acid as the main constituent in the acetone extracts of this species distinguishes it from other species in the Boesenbergia genus, which have a different compound composition. Given the abundance and diversity of the chemical composition in the rhizome essential oil and acetone extract, their antimicrobial activities against a wide range of Gram-positive and Gram-negative strains, as well as Candida yeast, have been elucidated. These findings will improve our understanding of the intricate relationship between the chemical composition of this newly discovered species and its biological effects. They will also facilitate the investigation of its potential applications, particularly in the fields of medical and food sciences.
Financial support
No financial support has been provided for this work





















Список литературы
Список использованной литературы появится позже.