An investigation into the acute and sub-chronic toxicityof ethanol extract of Ardisia sylvestris Pitard leaves in vivo
Aннотация
Background: The global rise in the usage of herbal medicine has prompted a critical examination of its safety. Despite widespread assumptions of safety, there remains a need for thorough toxicity evaluations to identify potential risks associated with herbal extracts. In traditional Vietnamese medicine, Ardisia silvestris is commonly used for various ailments, yet toxicity assessments specifically focusing on its leaves are lacking. The aim of the study: To assess the acute and sub-chronic toxicity of ethanol extract from A. silvestris leaves (EEAS) in mice via oral administration at varying doses. Materials and methods: This study employed two experimental models to assess toxicity: acute toxicity testing and sub-chronic toxicity testing. Each model comprised six groups, with six mice per group. The control group and the satellite control group received the vehicle, while the test groups and satellite test groups were administered EEAS at doses ranging from 1000 to 5000 mg/kg b.w (in acute toxicity testing) and from 100 to 500 mg/kg b.w (in sub-chronic toxicity testing). All treatments were conducted over 14 and 90 days, following which satellite groups were further monitored for 28 days after cessation of extract administration. Results: The acute toxicity evaluation revealed dose-dependent adverse effects such as elevated respiration rates, increased locomotor activity, sedative effects, and seizures in the initial hours post-administration. However, no mortality was recorded, with observed signs gradually diminishing (p<0.05) over the study period. In acute and sub-chronic tests, significant reductions in food and water intake were noted at higher doses (p<0.05), along with alterations in hematological and biochemical parameters (p<0.05) indicative of physiological stress. Organ weight assessments and macroscopic observations also indicated significant alterations in vital organ morphology at higher doses (p<0.05). Histopathological examinations revealed tissue damage and inflammatory infiltrations in the heart, liver, and kidneys. Urinary parameters displayed significant alterations (p<0.05), particularly in specific gravity, pH, and ketone levels. Nonetheless, these effects were temporary and reversible upon discontinuation of EEAS (p>0.05). Conclusion: Overall, while EEAS exhibited dose-dependent acute and sub-chronic toxicity effects, most adverse outcomes were reversible. This holds significant implications for assessing the safety of EEAS, indicating that the effects are manageable and do not result in prolonged or irreversible consequences.
Ключевые слова: acute toxicity, rodent model, sub-chronic toxicity, safety assessment, toxicological profile
К сожалению, текст статьи доступен только на Английском
Introduction. Since ancient times, plants have been utilized for medicinal purposes in various indigenous healthcare systems. Presently, herbal medicine is gaining popularity worldwide, especially in developing countries where medicinal plants are abundant and cost-effective [1]. While many people perceive natural remedies to have fewer side effects compared to synthetic drugs, the widespread belief that herbal medicine is entirely safe and devoid of adverse effects is not entirely accurate. In reality, several studies have uncovered potential harmful side effects associated with medicinal plants. It appears that using herbal remedies without assessing their toxicity could pose health risks. Therefore, it is crucial to investigate the potential adverse effects of herbal treatment methods [2]. Acute and sub-chronic toxicity tests are employed to assess chemicals' potential hazards and safety. These experiments involve evaluating the effects of a single dose or repeated doses of a chemical on animals and are often utilized to identify potential risks and manage hazards associated with chemical production, handling, and utilization [3]. Acute toxicity testing involves administering a single dose of a chemical to assess the severity of its harmful effects on animals. In contrast, sub-chronic toxicity testing entails administering repeated doses of the chemical to evaluate its potential for long-term effects on target tissues or organs. These experiments yield crucial information for identifying hazards and managing risks [4].
The genus Ardisia, comprising approximately 500 species, stands as the largest within the Myrsinaceae family. Ardisia species are widely distributed across tropical and subtropical regions worldwide and have been employed in the treatment of various ailments including cancer, hypertension, menstrual disorders, diarrhea, and among others. With their rich chemical composition, the isolation and identification of 111 different compounds from Ardisia species, including saponin triterpenoids, quinones, coumarins, depsipeptides, flavonoid quinones, polyphenols, saponin triterpenoids, isocoumarins, and alkylphenols, exhibiting anti-inflammatory, antibacterial, antioxidative properties, etc., have been reported [5]. Ardisia crispa (Thunb.) A. DC. (Primulaceae family) is a commonly used species in traditional folk medicine practices. In Thailand, its roots are utilized in the treatment of fever, inflammation, pain, joint disorders, and improvement of blood circulation, and combined with other plants to alleviate menstrual pain in women, while its leaves when crushed and applied to wounds, serve as a detoxifying remedy for snake or scorpion bites. In the Indochina region, extracts from the roots are employed to address chest-related issues, whereas in Taiwan, it is utilized as a diuretic and antidote. These traditional applications have garnered significant attention for their potential in modern medicine [6]. In traditional Chinese medicine, Ardisia japonica has been noted for its various benefits, including its potential to clear lung congestion, alleviate cough, stimulate blood circulation, and treat circulatory disorders [7]. Additionally, in traditional Vietnamese medicine, Ardisia silvestris (A. silvestris) has been reported to possess photoprotective properties by modulating the activation of protein kinases through scavenging free radicals, enhancing the expression of hyaluronic acid synthase-1, and increasing the production of occludin and transglutaminase [8]. However, to the best of our knowledge, despite the widespread use of A. silvestris in traditional folk medicine, there has been no study evaluating the toxicity of its leaves. Therefore, this study aims to determine the acute and sub-chronic toxicity via oral administration of ethanol extract from A. silvestris leaves in mice, with the hope that these findings will provide crucial information regarding the safety of this extract.
The aim of the study. Assess the acute and sub-chronic toxicity of orally administered ethanol extract from A. silvestris leaves in mice, aiming to provide essential safety data for this extract.
Materials and Methods
Plant material collection and ethanol extract preparation
Fresh leaves of A. silvestris were collected in An Toan commune, An Lao district, Binh Dinh province, Vietnam, from August to October 2023. A voucher specimen (AS051023VST) was deposited in the herbarium of the Department of Biotechnology, Institute of Biotechnology and Food Technology, Ho Chi Minh City University of Industry, Ho Chi Minh City, Vietnam. Leaves free from pests and diseases were carefully selected and cleaned before use. The leaves were air-dried in a cool place for two days, followed by further drying in a Memmert UN 110 drying oven (Memmert, Germany) at 60°C until the moisture content reached <12%. Subsequently, the dried plant material was cut into small pieces, ground into fine powder, and stored in moisture-proof bags at room temperature for use in subsequent experiments.
Extraction was carried out at room temperature using 70% ethanol, with 200 g of leaf powder per liter of ethanol. The filtrate was concentrated in a rotary evaporator RV 10 Digital V-C (IKA, Germany) at 40°C. The resulting ethanol extract of A. silvestris leaves (designated as EEAS) weighing 42 g was stored at 4°C until further use.
Phytochemical screening of ethanol extract A. silvestris leaves
In the investigation of ethanol extraction from A. silvestris leaves, botanical analysis was conducted to ascertain the presence of primary and secondary metabolites. The analytical approach adhered to standards outlined by Nhung et al., with minor adjustments made to ensure accuracy and relevance [9]. A detailed procedural outline is depicted in Table 1.
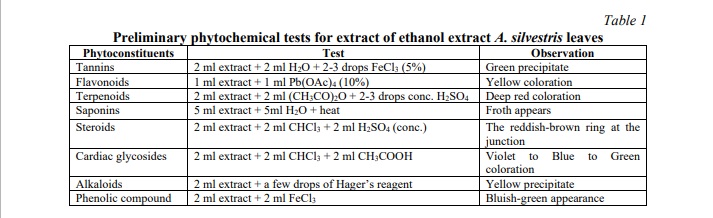
Phytochemical characterization of ethanol extract A. silvestris leaves
Determining the total phenolic content: The total phenolic content was determined using the Folin-Ciocalteu colorimetric method as described by Nhung and Quoc with some modifications [10]. Specifically, 0.3 ml of the extract was combined with 2.25 ml of Folin-Ciocalteu phenol reagent. After incubating for 5 minutes, 2.25 ml of 6% sodium carbonate solution was added to the mixture and left at room temperature for 90 minutes. Subsequently, the absorbance of the solution was measured at 725 nm. A standard curve of gallic acid was also established in the range of 0-200 μg/ml using a similar procedure. The results were expressed as milligrams of gallic acid equivalent (GAE) per gram of extract.
Determining the total flavonoid content: The total flavonoid content was determined using the aluminum chloride colorimetric method as described by Nhung and Quoc with specific adjustments [10]. Quercetin was used as the reference standard, and a standard curve for quercetin was generated in the concentration range of 0-200 μg/ml. For this analysis, 0.5 ml of the extract and 0.5 ml of the standard solution were placed into separate test tubes. To each tube, 10% aluminum chloride (0.1 ml), 1 M potassium acetate (0.1 ml), 80% methanol (1.5 ml), and distilled water (2.8 ml) were added and thoroughly mixed. A blank sample was prepared similarly, replacing 0.5 ml of the extract or standard solution with distilled water and substituting aluminum chloride with distilled water. All tubes were incubated at room temperature for 30 minutes, then the absorbance was measured at a wavelength of 415 nm. The concentration of flavonoids was expressed as milligrams of quercetin equivalent (QE) per gram of extract.
Experimental animals
Healthy Swiss albino mice, with an average weight of 29±2 grams and aged 6 to 7 weeks, were procured from the Pasteur Institute, Ho Chi Minh City, Vietnam. All mice were housed in glass cages measuring 60×30×30 cm (6 mice/cage) and maintained under standard laboratory conditions. This environment included a temperature of 24±2°C, humidity levels between 55-60%, and a 12-hour light/dark cycle. The cages were lined with wood shavings treated with Effective Microorganisms (EM) to eliminate bacteria and odors and were regularly changed every 2-3 days. The mice were cared for at the breeding facility of the Eastern Agriculture and Food Company, District 12, Ho Chi Minh City. The acclimatization process lasted for 7 days. We provided standard rodent food and free access to water for the mice. Throughout our research, we rigorously adhered to ethical principles concerning animal welfare, as stipulated by the unified ethical standards and the Helsinki Declaration on animal research [11].
Acute toxicity testing
This study adhered to the direct experimental procedure outlined by the Organisation for Economic Co-operation and Development (OECD) guidance document number 425 [12]. A total of 24 mice were divided into four test groups, each consisting of six mice. The dosage of extract used to assess acute toxicity was determined based on the method of calculation known as "dose per unit body weight" [13]. The experimental groups, including EEAS1000, EEAS3000, and EEAS5000, were administered a single dose of the corresponding extract at 1000, 3000, and 5000 mg/kg body weight, respectively. Additionally, the EEAS5000 satellite group also received a single dose of 5000 mg/kg body weight extract. Both the normal control group and the satellite control group received distilled water throughout the treatment period. The experimental and control groups were continuously monitored for 14 days. However, following this period, the satellite control group and the EEAS5000 satellite group were monitored for an additional 28 days. Before treatment, the body weight of each mouse was measured to determine the appropriate dosage. Mice were fasted and deprived of water overnight before administration of the extract. Each mouse received a single dose of the extract via gastric intubation within 24 hours. Clinical manifestations of toxicity were monitored and recorded immediately within the first hour post-administration of the EEAS dosage, followed by assessments every 2 hours over 24 hours, and continued daily for the subsequent 14 days. Clinical toxicity indices such as mortality rate, general behavior, body weight, food, and water intake, as well as urine analysis of the treated mice, were recorded weekly. During the final observation period, parameters including hematological indices, serum biochemical profiles, relative organ weights, and histopathological analyses of the organs were also analyzed and evaluated.
Sub-chronic toxicity study
The sub-chronic toxicity study of EEAS was conducted following OECD 408 guidelines [14]. EEAS at doses of 100, 300, and 500 mg/kg body weight were selected and administered orally daily for 90 days to three different groups of mice, designated as EEAS100, EEAS300, and EEAS500 groups, respectively. Throughout the treatment period, all experimental animals were observed weekly for any abnormal clinical signs, body weight changes, food, and water consumption, as well as urine analysis done in 90 days. The satellite control group and the EEAS500 satellite group were further monitored for an additional 28 days after the end of the experimental period. After the study, the mice were euthanized and dissected to analyze and evaluate hematological parameters, serum biochemical profiles, relative organ weights, and organ histopathology.
In each toxicity testing model, 24 mice were randomly assigned to 4 groups, with all mice housed in separate cages. Individual identification was established by permanent tail marking using a marker pen [15]. To achieve observational objectivity, all observations and data recordings were conducted blindly. Research personnel recorded data without knowledge of animal group assignments. Following EEAS administration, each mouse was continuously monitored for the first hour, then every 2 hours for 24 hours, and subsequently once daily to days 14 and 42. All animals were clinically observed daily for signs of toxicity and mortality rates. Visual observations on skin and fur characteristics, eyes, respiratory patterns, the autonomous nervous system feature such as salivation, diarrhea, and urination, central nervous system characteristics such as tremors, relaxation, gait, and posture, and any other abnormal behaviors were conducted once daily [16].
Body weight assessment
The body weight of each mouse was measured using a Sartorius Entris 224i-1S electronic balance (Sartorius, Germany) during the experiment. Mouse body weight was carefully monitored once before drug administration, once weekly throughout the study, and once on the sacrifice day. Weight gain (WG) was calculated using the formula:
Food and water consumption
Data about food and water intake was recorded before administration to the mice. All remaining food and water were collected and weighed at the end of each day. Daily food and water intake were calculated using the following formula:
Food consumption (g) = Initial food consumption (g) - Remaining food consumption (g)
Water intake (ml) = Initial water intake (ml) - Remaining water intake (ml) [17]
Hematological and biochemical parameters
Following the experimental period, all surviving animals were subjected to overnight fasting, and blood samples were collected via the retro-orbital sinus method. Blood was divided into two groups of tubes, with half of the tubes containing an anticoagulant, ethylene diamine tetraacetic acid (EDTA), for hematological analysis, while the remaining half without anticoagulant was used for biochemical analysis. Estimates of hemoglobin (HGB), hematocrit (HCT), total white blood cell count (WBC), red blood cell count (RBC), and platelet count (PLT) were analyzed using an automated hematology analyzer (Sysmex-RX, 21, Japan). Blood samples in tubes without anticoagulant were allowed to clot, and serum was obtained by centrifugation using an electric centrifuge machine (Humax-K, Germany) [18]. Creatinine (CRE) and urea (URE), uric acid (URA), total protein (TP), glucose (mg/ml), triglyceride (mg/ml), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were analyzed using an automated clinical chemistry analyzer (Auto-Lab 18, Italy).
Assessment of relative organ weight
On the final examination day, mice were euthanized by CO2 inhalation and dissected for macroscopic examination. Organs such as the heart, liver, and kidneys were carefully excised from the body and weighed. The specimens were then fixed in a 10% formalin solution to achieve histopathological sections. The relative organ weight (ROW) of each animal was calculated as follows:
 [19]
[19]
Histological assessment
The heart, liver, and kidneys were harvested and fixed in neutral buffered formalin (NBF) 10%. Following fixation, tissue sections were rinsed with water and dehydrated through graded alcohols (70%, 90%, and absolute alcohol) and cleared twice in xylene. Subsequently, tissues were embedded in paraffin blocks to form paraffin blocks. Tissue blocks were sectioned into 4 μm-thick ribbons using a microtome. Tissue sections were mounted on glass slides and stained routinely with hematoxylin (H) and eosin (E). Then, the slides were dehydrated in 50%, 70%, 95% alcohol, and absolute alcohol. Dehydrated sections were cleared in xylene. DPX and coverslips were applied and fixed on microscope slides. Observations were made under a light microscope to detect any histological changes. Following examination, photomicrographs of selected tissue sections were captured at a magnification of ×200 using an integrated digital camera (Evos XL, USA) [17].
Urinalysis
Before urine collection, each mouse was individually placed in a sterilized glass cage for 12 hours to obtain urine samples. Each cage was equipped with water but no food. Fresh urine from each mouse in the glass cage was collected. Urine samples were analyzed and evaluated for specific gravity, pH, ketone, protein, glucose, and occult blood (red blood cells and white blood cells) using dipsticks, a semi-automated urine analyzer (Meditape UC-1000, Kobe, Japan), and a microscope [17].
Statistical analysis
The results are presented as mean values ± standard deviation. Differences between the treatments were determined using one-way analysis of variance (ANOVA) followed by Fisher's LSD test, utilizing Stagraphics Centurion XVI software (Statpoint Technologies Inc., Warrenton, Virginia, USA), with the statistical significance threshold set at p<0.05.
Results and discussion
Qualitative phytochemical analysis
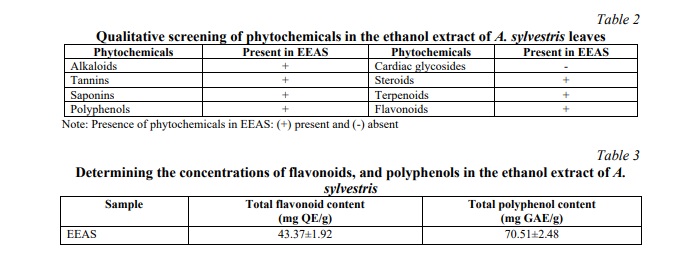
In the extract of A. sylvestris leaves (EEAS), qualitative phytochemical screening revealed the presence of alkaloids, tannins, saponins, polyphenols, steroids, terpenoids, and flavonoids, while cardiac glycosides were absent (Table 2). Quantitative analysis of phenolic and flavonoid contents in EEAS yielded results as presented in Table 3. Specifically, the total phenolic content (TPC) was determined to be 70.51±2.48 mg GAE/g DW, whereas the total flavonoid content (TFC) was found to be 43.37±1.92 mg QE/g DW. These values provide insightful information regarding the concentration of phenolics and flavonoids within EEAS.
Ardisia silvestris P. is a member of the Ardisia genus, belonging to the Myrsinnaceae family, with a long history of traditional medicinal use due to the presence of bioactive compounds. A. silvestris exhibits various valuable biological activities including antibacterial, antifungal, antiviral, anti-inflammatory, analgesic, antioxidant, anti-diabetic, anti-osteoporotic, neuroprotective, hepatoprotective, and notably, highly effective anticancer properties [20]. To assess the potential toxicity of A. silvestris, a commonly used species in traditional medicine, both acute and sub-chronic toxicity are investigated. Current research reveals that the ethanol extract of A. silvestris leaves (EEAS) contains phytochemical compounds, notably exhibiting high concentrations of polyphenols and flavonoids. These findings align with those of Huynh et al., who utilized petroleum ether, ethyl acetate, ethanol, and water extracts and reported elevated TPC and TFC values in A. silvestris leaves [20]. The presence of alkaloids, tannins, saponins, polyphenols, steroids, terpenoids, and flavonoids in the extract signifies a rich concentration of phytochemicals with potential therapeutic benefits. These compounds are associated with various pharmacological properties such as antioxidant, anti-inflammatory, antibacterial, and anticancer activities. The significant levels of phenolic and flavonoid contents in the extract indicate its potential efficacy. Phenolics and flavonoids are renowned for their antioxidant properties, playing a crucial role in neutralizing harmful free radicals and reducing oxidative stress-related damage in the body. Furthermore, flavonoids are linked to anti-inflammatory effects, cardiovascular protection, and modulation of cellular signaling pathways involved in disease progression [21]. Despite the promising therapeutic potential conferred by the botanical chemical composition and high levels of phenolic and flavonoid content in the extract, careful consideration of dosage and duration of use is paramount to mitigate potential adverse effects. Alkaloids, while often possessing therapeutic properties, can also exhibit toxic effects at high doses. Some tannins and saponins may cause gastrointestinal irritation and other adverse reactions when consumed excessively. Steroids, terpenoids, and flavonoids, although offering potential health benefits, can also have toxic effects on various organ systems if consumed in excessive quantities, potentially causing gastrointestinal discomfort and interfering with nutrient absorption in large amounts. The high concentration of phenolic and flavonoid contents, while indicative of the extract's therapeutic potential, also raises concerns about potential toxicity, especially with prolonged or excessive consumption. Specifically, phenolic compounds may induce cellular toxicity at high doses, while flavonoids can interfere with certain enzyme processes and drug metabolism pathways, leading to adverse reactions [22]. Therefore, comprehensive toxicity studies of EEAS are necessary to assess the safety profile of the extract and ensure its suitability for therapeutic purposes.
Behavior evaluation
Observation of acute toxicity behavior in experimental animals following administration of the extract at concentrations of 1000, 3000, and 5000 mg/kg b.w revealed elevated respiration rates within the first hour in the treated groups, accompanied by increased body locomotor activity within the initial 2 hours. Sedative effects and drowsiness were noted across all extract-treated groups during the first 4 hours. Seizures and tremors were observed in animals treated with the extract consistently within the initial 4 hours, particularly in the 5000 mg/kg b.w dose group, with itching and tremors persisting up to 24 hours. Occasionally, itching was observed in the EEAS3000, EEAS5000 group, and satellite EEAS5000 group during the first week of the study. No mortality was recorded throughout the study period. However, signs gradually diminished after 14 days of observation, with complete disappearance by day 42 in the satellite EEAS5000 group.
The toxic effects of drugs on vital organs of the body are manifested through clinical signs and symptoms, which are among the primary observations of various toxicity indices [23]. In the initial hours following exposure to the extract, an increase in respiration rate and heightened body movements are observed, indicative of mental stimulation or activation of components within the nervous system. The effects of EEAS occur either directly on portions of the central nervous system or through mechanisms akin to receptor stimulation, eliciting neural responses. The observed soothing and sedative effects stem from EEAS components capable of influencing the central nervous system, diminishing alertness, and promoting drowsiness. Convulsions and tremors in mice signify nervous system stimulation or aberrant neuronal activity. EEAS components impact the electrical activity of nerve cells, resulting in seizures and tremors. Sensations of itchiness and stinging denote the body's reaction to components within the extract [24]. Following initial exposure to the toxic extract, the animal body processes the toxin through metabolic pathways, urinary excretion, and other mechanisms. As the toxic load diminishes within the body, clinical symptoms gradually abate. Subsequently, there may be a phase where the toxic extract accumulates within the organism, precipitating symptomatic manifestations. However, by the 42nd day, the body has effectively eliminated the toxin, resulting in a gradual resolution of clinical manifestations. Of particular note is the adaptive capacity of the animal body to endure the presence of toxins over some time. Once adaptation occurs, symptomatic manifestations also diminish as the body's inherent defense mechanisms stabilize endocrine and nervous system function. The detoxification process may extend over some time and does not occur immediately. By day 14, a substantial portion or the majority of the toxin may have been eliminated, with complete eradication achieved by day 42 [23].
Body weight changes
Throughout the study period, both control and ethanol extract of A. sylvestris leaves (EEAS) treated groups exhibited a gradual increase in body weight in both acute and sub-chronic toxicity experiments, as depicted in Table 4 (p<0.05). The variation in body weight gain (p<0.05) between EEAS-treated mice and the control group was observed consistently during the study phase (Fig. 1). Upon concluding acute and sub-chronic toxicity assessment, the body weight and weight gain of mice in satellite groups (Satellite EEAS5000 group and Satellite EEAS500 group) showed no significant difference (p>0.05) compared to the satellite control groups (Table 4, Fig. 1).
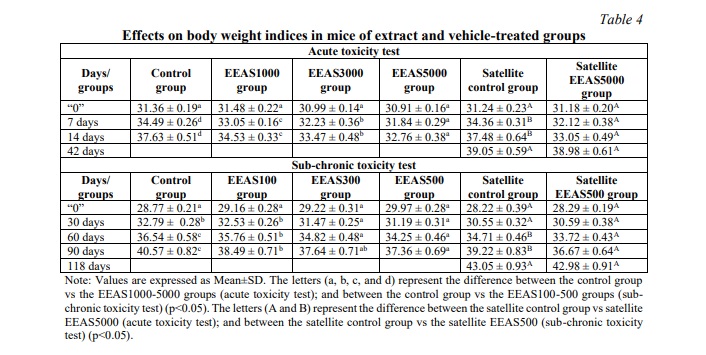
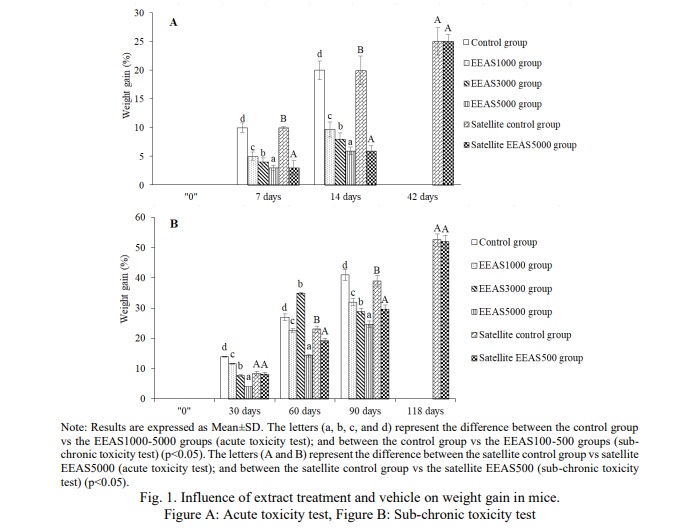
In toxicity experiments, changes in body weight serve as an indicator of potential adverse effects of the administered extract. Body weight alterations signify nutritional status changes and the overall health of the animals, serving as markers for the influence of external factors such as stress, toxins, or environmental conditions. Variations in animal body weight serve as early manifestations of the toxic effects of the administered extract. Decreases in weight may indicate compromised health, nutritional disorders, bodily injury, or intricate and sensitive signs following exposure to the extract. Conversely, weight gain suggests enhanced nutrient absorption or increased cellular tissue production, while also signaling allergic reactions or metabolic stress [25]. In the current study, both the group treated with ethanol extract from A. sylvestris leaves (EEAS) and the control group exhibited a gradual increase in average body weight within normal ranges. The weight gain between the control group and the EEAS-treated group at doses up to 5000 mg/kg b.w and 500 mg/kg b.w did not show significant differences, particularly in the satellite groups. These results indicate that both the EEAS-treated group and the control group demonstrated a normal trend of increasing average body weight. EEAS did not induce significant alterations in body weight compared to the control group under the established study conditions. EEAS may not exert significant harmful effects on the body weight of experimental animals.
Food and water intake
During the experiment, weekly recordings of food and water consumption were conducted. The results revealed a significant reduction in food and water intake at doses of 3000 and 5000 mg/kg body weight (in acute toxicity testing) and 300 and 500 mg/kg body weight (in sub-chronic toxicity testing) compared to the control group (p<0.05) (Tables 5 and 6) on days 7 and 14. However, no significant differences in food and water intake were noted between the Satellite control group and the Satellite EEAS5000 and Satellite EEAS500 test groups on days 42 (in acute toxicity testing) and day 118 (in sub-chronic toxicity testing) (p>0.05) (Tables 5 and 6).
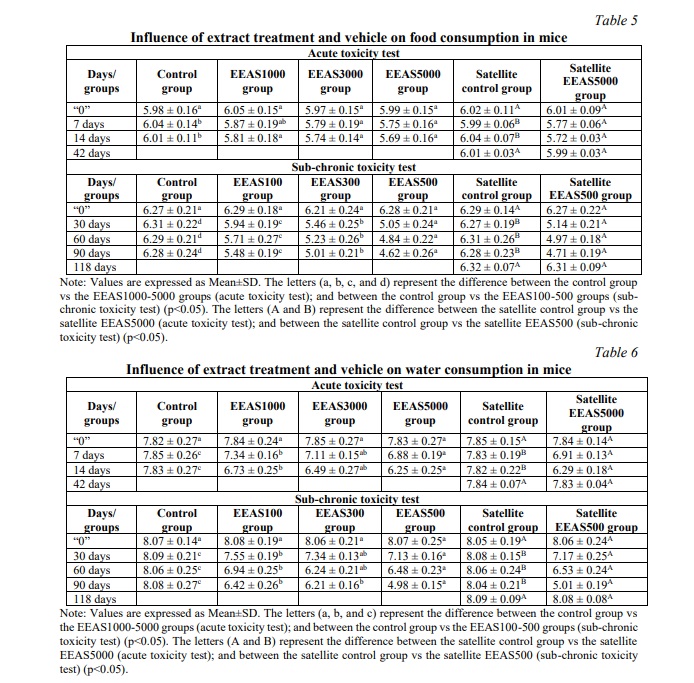
Food and water intake play crucial roles as fundamental indicators of health and overall physiological status in an organism. Changes in consumption patterns may reflect alterations in physiological status, such as decreased appetite, and digestive or metabolic disorders, resulting from exposure to toxic substances [26]. Reductions in food and water intake following exposure to a potentially toxic substance can indicate adverse effects on the organism, including impacts on the digestive system, liver, kidneys, or central nervous system, among others. Changes in food and water intake serve as endpoints for toxicity assessment, providing vital information for evaluating the overall impact of the extracted substance and its safety profile. Monitoring food and water consumption over time allows for the assessment of dynamic changes in toxicity responses. Recovery or adaptation models following cessation of exposure provide insightful information on the reversibility of harmful effects and the organism's recovery capabilities [27]. The impact of EEAS on food and water consumption reflects the biological effects of this extract on the body. The reduced intake of food and water in the high-dose EEAS groups suggests that EEAS may affect or decrease the absorption of food, water, or nutrients in the body. This outcome may be due to the negative effects of EEAS on the digestive or endocrine systems, influencing metabolism and energy expenditure, or changes in taste perception and appetite. Changes in food and water consumption provide valuable information about the biological effects of EEAS on the body, aiding in understanding its potential to address health and environmental issues. However, no significant differences in food and water consumption were observed between the Satellite control group and the Satellite EEAS5000 and Satellite EEAS500 test groups on day 42 (in acute toxicity testing) and day 118 (in sub-chronic toxicity testing), indicating that the effects of EEAS on food and water consumption may be temporary and reversible after discontinuation of its use. This provides important insights into the independence and recovery capabilities of the body after exposure to EEAS.
Hematological and biochemistry analysis
The effects of administering the ethanol extract of A. sylvestris leaves (EEAS) orally on hematological parameters are summarized in Table 7. In acute and sub-chronic toxicity experiments, we observed a significant decrease in the EEAS-treated groups (p<0.05) compared to the control in terms of red blood cell count (RBC), hemoglobin concentration (HGB), and hematocrit levels (HCT). Additionally, white blood cell count (WBC) and platelet count (PLT) significantly increased (p<0.05) during the 14-day (acute toxicity) and 90-day (sub-chronic toxicity) experimental periods. Table 8 illustrates the fluctuation of biochemical parameters in mice treated with EEAS compared to the control group. The results showed a significant decrease (p<0.05) in the levels of total protein (TP), glucose (GLU), and triglycerides (TRI) in the EEAS-treated groups at various doses compared to the control group. Meanwhile, the concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), uric acid (URA), blood urea nitrogen (BUN), and creatinine (CRE) significantly increased (p<0.05) compared to the control group throughout the 14 and 90-day periods. These current findings indicate that oral administration of EEAS may impact hematological and biochemical parameters when exposed to it. However, in the satellite groups, no significant changes (p>0.05) in hematological and biochemical parameters were observed after 42 and 118 days of follow-up (Tables 7 and 8). Specifically, after 42 days of follow-up in the acute toxicity model and after 118 days of follow-up in the sub-chronic toxicity model (i.e., 28 days after discontinuation of EEAS), there were no significant differences (p>0.05) in hematological and biochemical levels between the EEAS5000 and EEAS500 satellite groups compared to the control satellite group. These results indicate that the effects of EEAS on hematological and biochemical parameters are temporary and reversible after discontinuation of the extract.
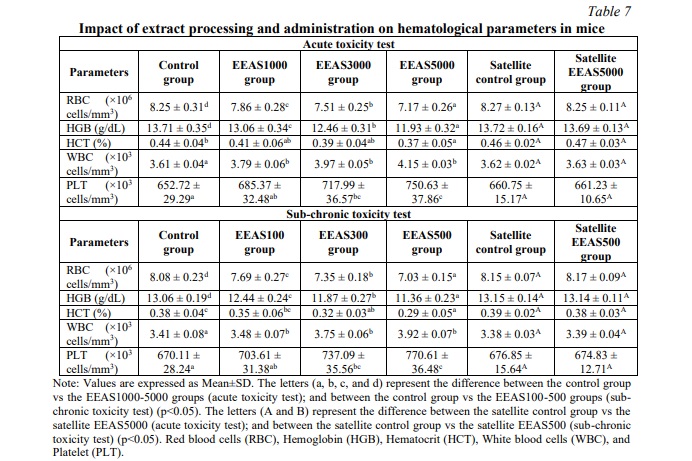
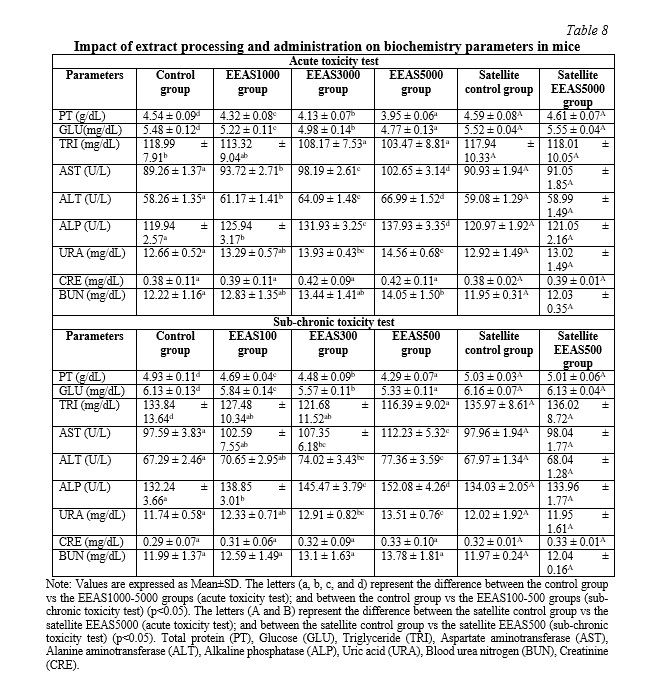
Nutrients and foreign substances are primarily transported throughout the body via the bloodstream. Therefore, blood components such as RBCs, WBCs, PLTs, HCT, and HGB are often exposed to toxic substances. The results regarding the hematological parameters of the groups treated with EEAS in this study show a significant decrease in RBC, HGB, and HCT compared to the control group. This could be explained by the effects of active components in the extract on the production and maintenance processes of red blood cells in the body. The components in the extract may influence the production of red blood cells in the bone marrow or increase the destruction of red blood cells in the immune cell system, while also affecting the flexibility of the red blood cell membrane, leading to increased destruction and decreased lifespan of red blood cells, thereby reducing the number of RBCs. The decrease in RBC count also leads to a decrease in hemoglobin concentration and hematocrit level in the bloodstream. Additionally, the WBC and PLT counts increased significantly compared to the control group after using the extract. This increase is due to the body's immune response to the components in the extract. Upon exposure to the extract, the immune system stimulates the production and release of white blood cells and platelets from specific cell sources such as the bone marrow and spleen, under the regulation of hormones and growth factors, thereby increasing the number of WBCs and PLTs to protect the body against harmful agents [28]. Biochemical parameters are fundamental diagnostic criteria in clinical practice. They indicate adverse effects caused by substances. Biochemical analyses are used to identify, detect, and describe the harmful effects caused by toxic compounds. These analyses are also crucial for assessing specific target organs affected by toxic compounds and provide valuable information to understand them. The values of URA, URE, and CRE are parameters used to assess kidney damage. Liver function can be evaluated by the levels of markers, including PT, GLU, TRI, ALT, AST, and ALP. The liver and kidneys, involved in xenobiotic elimination, are sensitive organs that can be altered by substances, including plants and drugs. After using EEAS, the levels of PT, GLU, and TRI significantly decreased compared to the control group, indicating the impact of active components in the extract on metabolic processes and nutrient storage in the body. The components in the extract affect protein synthesis, reduce glucose absorption from the intestine, and inhibit triglyceride synthesis and storage in the body. Moreover, they also inhibit the activity of enzymes involved in protein, glucose, and lipid metabolism and affect the transformation of fat cells and their regulatory mechanisms [25]. Furthermore, the components in EEAS stimulate the growth of liver cells and intestinal mucosal cells, leading to the release of liver and intestinal enzymes. Additionally, EEAS also promotes the development of kidney cells, reducing renal filtration function and increasing the production of catabolites. As a result, the increased production and release of these enzymes into the bloodstream disrupt the body's water and electrolyte balance, leading to significant increases in AST, ALT, URE, CRE, and URA levels in the blood of mice after EEAS administration compared to the control group [28]. However, after 42 days of observation in the acute toxicity model and 118 days in the sub-chronic toxicity model (equivalent to 28 days after discontinuation of EEAS), there were no significant differences (p>0.05) in hematological and biochemical parameters between the EEAS5000 and EEAS500 satellite groups compared to the control satellite group. Therefore, the impact of EEAS on hematological and biochemical indices appears to be temporary and reversible after discontinuation of the extract.
Relative organ weight
After the experimental phase, the relative organ weights (ROW) of the mouse's vital organs (heart, liver, and kidneys) were assessed, with results depicted in Fig. 2. Overall, in both the acute toxicity model (Fig. 2A) and the sub-chronic toxicity model (Fig. 2B), the ROW of the mouse's heart, kidneys, and liver in groups treated with different doses of EEAS all showed significant statistical increases compared to the control group (p<0.05) after 14 and 90 days of observation. Continued monitoring for an additional 28 days (without extract usage) showed that the ROW of the heart, kidneys, and liver in the EEAS5000 and EEAS500 satellite groups were nearly equivalent to the control satellite group (p>0.05).
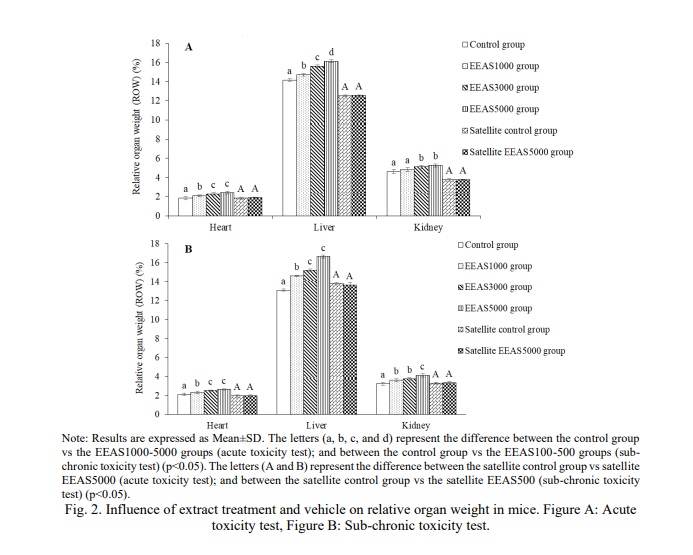
In toxicity experiments, the relative organ weight (ROW) of the heart, liver, and kidneys is commonly used to assess the impact of toxic substances on the body. Changes in organ weight can reflect alterations in the structure and function of these organs following exposure to toxins, while also providing valuable insights into the extent of organ damage and recovery after cessation of exposure to the toxic substance. In the current study, the increase in relative organ weight (ROW) of the heart, liver, and kidneys following the use of the extract reflects the body's response to the extract or its active components. The body typically employs reactive mechanisms to adapt to the impact of new substances, including increasing the size and activity of vital organs such as the heart, liver, and kidneys. The significant increase in liver ROW indicates enhanced hepatocyte proliferation or increased cellular organization to aid in the elimination of toxins or boost the production of liver enzymes to process them. The increase in kidney ROW after EEAS use signifies the body's response to enhance the filtration and elimination of harmful substances. The heart, responsible for pumping blood to nourish the body, exhibits an increased ROW as a response to the increased demand for oxygen supply to the body to deal with toxic substances [29]. Hence, the use of EEAS induces significant changes in the size of vital organs such as the heart, liver, and kidneys. These results demonstrate the body's response to the extract or its active components. However, over time, these changes appear to be transient and may potentially reverse upon discontinuation of the extract. This suggests that the effects of the extract on the body may not be long-lasting or deeply impactful on the structure and function of these organs in the long term.
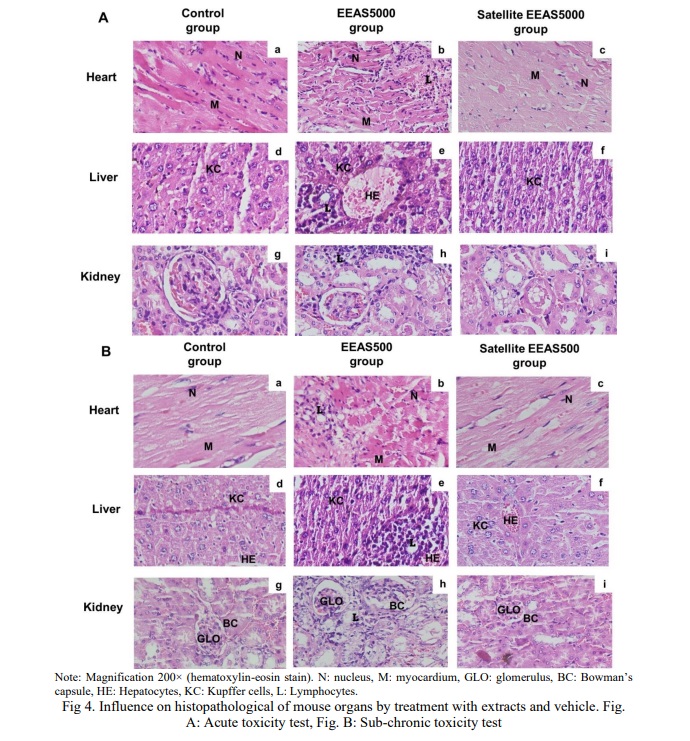
Macroscopic and histopathological structure of organs
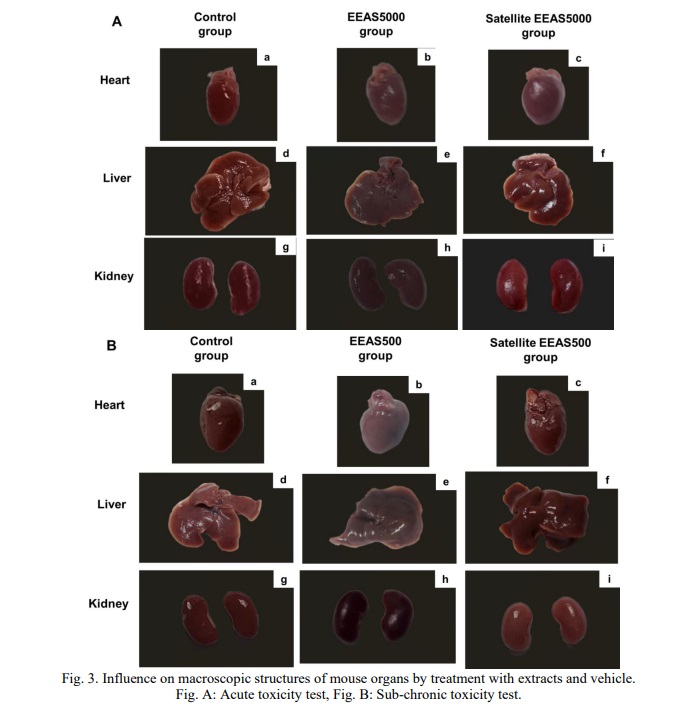
In the histopathological examination of the mouse heart treated with EEAS at doses of 5000 mg/kg b.w and 500 mg/kg b.w (Fig. 4Ab and Fig. 4Bb, respectively), alterations in the nucleus and myocardium were observed, along with the presence of lymphocyte infiltration. Similarly, the hepatic tissue structure exhibited changes (Fig. 4Ae and Fig. 4Be, respectively), characterized by disrupted cell arrangement, dilation of portal veins and central veins, and increased lymphocytic infiltration within the hepatic sinusoids. Likewise, renal tissue morphology was also affected (Fig. 4Ah and Fig. 4Bh, respectively), with distorted glomeruli and Bowman’s capsules, as well as infiltration of lymphocytes into Bowman’s capsules and renal tubules. The results indicate histopathological alterations in the heart, liver, and kidney tissues of mice treated with EEAS. These alterations include changes in cell morphology, such as lymphocyte infiltration, disruption of cell arrangement, and distortion of anatomical structures like glomeruli and Bowman’s capsules. These findings suggest potential adverse effects of EEAS on the cellular integrity and structural organization of vital organs. However, the tissue structure of the heart, liver, and kidneys in the EEAS5000 and EEAS500 satellite groups returned to near-normal levels, comparable to the control group. The presence of hepatic cells, hepatic sinuses, portal triads, and central veins (Fig. 4Ae and 5Be, respectively) appeared normal compared to the control (Fig. 4Ad and 4Bd). In the renal histopathological study, the structure of the renal corpuscles, renal tubules, and Bowman’s capsules in the EEAS5000 and EEAS500 satellite groups (Fig. 4Ai and 4Bi) did not differ from the control group (Fig. 4Ag and 4Bg). The myocardial tissue in the EEAS5000 and EEAS500 satellite groups (Fig. 4Ac and 4Bc) also exhibited intact structures with nuclei and myocardium, comparable to the myocardial tissue structure of the control group (Fig. 4Aa and 4Ba). Importantly, there was no longer evidence of lymphocyte infiltration in the tissues of these organs. The results indicate that following the use of the extract, the tissue structure of the heart, liver, and kidneys has largely returned to a normal state, comparable to that of the control group. This suggests that the extract may not induce significant alterations in the tissue structure of these organs and may not exert significant adverse effects on them.
Urinalysis
In the present study, Table 9 illustrates the changes in urinary parameters observed in mice treated with different doses of EEAS compared to the control group (p<0.05). Protein, glucose, and occult blood (red and white blood cells) were not detected in the urine of the experimental animals. However, specific gravity, pH, and ketone levels in the urine of mice treated with EEAS differed significantly from the control group (p<0.05) on days 14 and 90 of the two corresponding toxicity models. Nevertheless, in the EEAS5000 and EEAS500 satellite groups (groups monitored for an additional 28 days after drug administration), there was a significant decrease in specific gravity, pH, and ketone levels in the urine, with results nearly equivalent to the control satellite group (p>0.05).
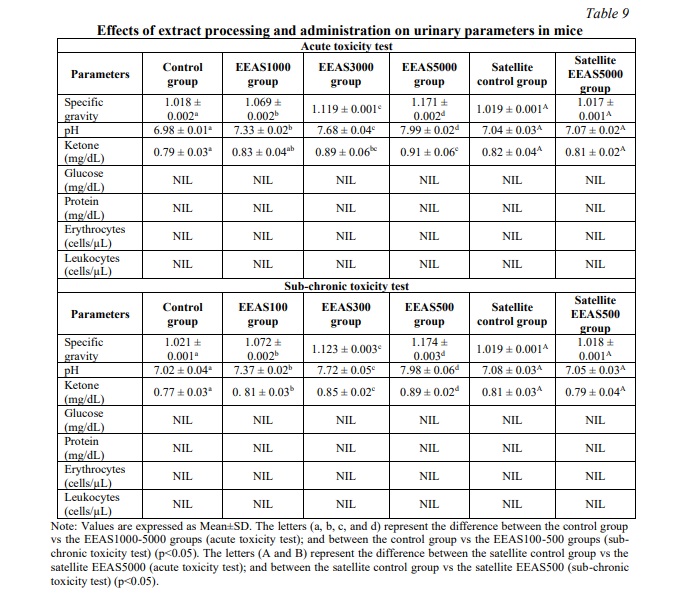
Long-term exposure to xenobiotics can have detrimental effects on various systems and organs in the body, including the nervous, endocrine, cardiovascular, and musculoskeletal systems, as well as organs such as the lungs, liver, and kidneys. Consequently, the liver and kidneys serve as the primary detoxification organs in the body. The process of xenobiotic detoxification in the body is intricate, with toxins being metabolized and eliminated through processes such as hydrolysis, oxidation-reduction, and detoxification, ultimately excreted via urine [30]. In the current study, significant increases in urinary specific gravity, pH, and ketone levels were observed in mice following the administration of the extract compared to the control group. Specific gravity serves as a measure of urine concentration, and the elevation may indicate dehydration or the presence of solutes increasing urine density, such as dissolved substances from the extract. The rise in pH is indicative of metabolic changes induced by the extract or its components. Additionally, the increased ketone concentration suggests enhanced fat metabolism or ketosis, possibly due to the metabolic influence of the extract on the body. These alterations collectively reflect changes in urinary composition and metabolic processes following extract administration [25]. In the satellite groups, the urinary-specific gravity, pH, and ketone concentrations have returned to baseline levels. This normalization could be attributed to the cessation of extract administration, allowing the body to restore normal metabolic processes and urinary parameters. The restoration of these urinary parameters indicates that any effects induced by the extract on urine composition and metabolism may be reversible upon discontinuation of use.
Conclusion. The study investigated the acute and sub-chronic toxicity of the ethanol extract of A. sylvestris leaves in vivo. The findings revealed acute toxicity symptoms, including elevated respiration rates, sedative effects, and seizures, which gradually diminished over time. Additionally, the extract affected body weight gain, food and water intake, hematological and biochemical parameters, relative organ weights, macroscopic and histopathological changes, and urinary parameters. However, these effects were temporary and reversible after discontinuation of the extract, suggesting potential safety concerns but also indicating the possibility of manageable toxicity levels with proper monitoring and management.
Financial support
No financial support has been provided for this work






















Список литературы
Список использованной литературы появится позже.