Coexistence of Urinary Incontinence and Osteoporosis in Females – A Systematic Review
Abstract
Background: Urinary incontinence (UI) is described as the involuntary leakage of urine and is a global problem. The younger age groups have the lowest prevalence (12.0%), while the oldest have the greatest (40.0%); however, there is a surge around the middle age. Osteoporosis (OP) is a medical and socioeconomic hazard characterized by the decrease of bone mass, strength throughout the body resulting in lower bone density and a higher risk of fractures. Females become more vulnerable to these conditions as they grow older. The aim of the study: To assess the available research and find links between coexistence of decreased bone health and urinary incontinence in females. Materials and methods: Electronic databases like, CINAHL, Embase, Trip Medical Database, Cochrane Library and Pub Med were the ones searched for relevant articles from January 2011 to January 2022. The PRISMA Statement for Systematic Reviews and Meta-Analysis was used to conduct this systematic review. Results: There were 416 results found in the databases after eliminating the duplicates and studies that were unrelated to the topic. The review included total of five studies and quality assessment was done by four reviewers. Most studies found a strongly significant link between osteoporosis and urinary incontinence, whereas one study found no association. Conclusion: In this study, the five most common associated risk factors were revealed to be menopause, obesity, smoking, physical inactivity, and hyperlipidemia. Based on recent studies a strong significant link was found between the two health conditions (OP and UI) and coexistence of both conditions was seen in females.
Introduction. Osteoporosis (OP) is a medical and socioeconomic hazard characterized by a systemic loss of bone mass, strength, resulting in lower bone density and a higher risk of fragility fractures [1]. The term "osteoporosis" was first used in French in the early 1820s to describe a pathological state of the bones, but it was only in the twentieth century that it entered the English medical language [2]. OP is frequently discovered until after the first clinical fracture. The initial signs of OP are usually acute back pain caused by a fracture or groin discomfort caused by a hip fracture [3]. Menopause and ageing lead to Type I and Type II OP respectively and are the commonest among the various forms of OP. Alcohol misuse, hypercortisolism, hyperthyroidism and hyperparathyroidism immobility are all secondary causes of OP [4].
Urinary incontinence (UI) is described as the involuntary leakage of urine and is a global problem that affects 13.9 percent of men and 51.1 percent of women [5]. Involuntary urine leaking from the urethra synchronous in response to exercise or elevated abdominal pressure without detrusor contractions is known as stress urinary incontinence (SUI). Urge urine incontinence (UUI), on the other hand, is involuntary leaking that is accompanied by an instant sense of urgency and is caused by involuntary detrusor contractions. Both urgency and effort cause mixed urinary incontinence (MUI) [6].
Old age, obesity, and smoking, as well as pregnancy and delivery, all appear to have a role in the disease. Women who are nulliparous have a lower frequency than those who are multiparous [5, 7]. The younger age groups have the lowest prevalence (12.0%), while the oldest had the greatest (40.0%). However, there is a surge around the middle age, with a frequency of 30.0% among women aged between 50-54 [8]. It’s a costly condition affecting women cross culturally in all age groups [9].
The pathophysiological mechanism for the higher UI incidence in women with osteoporosis is because of two factors. One of the primary for the higher prevalence of urinary incontinence is that because of osteoporosis there are changes in spinal curvature which further lead to vertebral compression or spinal compression fracture. These changes lead to persistent rise in intra-abdominal pressure, which pushes down on the pelvic floor muscle and weakens its fast as well as slow twitch fibers finally leading to urinary incontinence [10, 11]. The second point is that osteoporosis and pelvic floor diseases have the same etiology and pathophysiology. Both osteoporosis and UI are linked to sarcopenia, an age-related muscular condition that reduces muscle size and function and leads to additional loss of connective tissue tension such as irregularities in mass, matrix, and microstructure, weakening the pelvic floor's supportive strength and thereby causing UI. The control of oestrogen and selective oestrogen receptor modulators help to alleviate these detrimental processes [12].
Methods
Study design. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement for Systematic Reviews and Meta-Analysis, a 27-item checklist was used to conduct this systematic review [13].
Literature search. An electronic search of five databases i.e. Embase, PubMed, CINAHL, Trip Medical Database and Cochrane Library were performed from January 2011 to January 2022 to identify relevant studies. Besides, a Google Scholar search was done to find more related articles. To find related articles, a variety of keywords were utilized. Urinary incontinence; osteoporosis; bone density disorder; urine dysfunction; prevalence; association were used as search phrases. Only human participants were included.
Eligibility criteria. Studies were considered if: (1) the participants with UI of any type were part of the study; (2) women with OP irrespective of their age were included in the research;(3) publications were in English; (4) studies were published in previous 10 years. The authors were also contacted if there was any missing information in potentially relevant research. When the author did not answer or stated that he or she was unable to, the study was ruled out due to a lack of relevant data. Participants with neurological disorders and any other bone-related issues were ruled out. Any study that was a review or a randomised controlled trial (RCT) was excluded. Because of the inadequate information, conference abstracts were not considered for inclusion.
Study selection. The four authors (SP), (SB), (AP), (VS) assessed the abstracts of the studies separately at first. The studies chosen were ones relevant to the population examined, diagnoses of the patients, as well as the study's objective and conclusion. To determine eligibility, full articles were obtained, and bibliography was checked for related literature. Any disagreement amongst the reviewers was settled through mutual conversation.
Data extraction and synthesis. The four reviewers (SP), (SB), (AP), (VS) worked separately to extract data from each research, and recorded the information.
Quality assessment. The “Guidelines for critical appraisal for the health research literature: prevalence or incidence of a health problem” by Loney et al. was used for the quality assessment of included studies [14]. This tool consists of eight things, each of which was awarded one point. The “Yes” (1 point) and “No” (0 points) are the options for this tool. The item was given a “No” score (0 points) when the information was not enough to reach a conclusion. Quality ratings varied from 0 to 8, with 0 being the lowest and 8 being the highest. When the score was fewer than four points (<4), the study was classed as poor quality, four to six points as moderate quality and seven or more points (>7) as high quality.
Results
Study characteristics. The research work included were chosen individually by the four authors (SP, SB, AP, VS) taking inclusion and exclusion criteria in consideration.
There were 416 results found in the databases. After eliminating the duplicates and studies that were unrelated to the research question, six studies were included. One potential research project was turned down because the author failed to provide further information in the methodological area. This review includes five studies, which are listed below in the Table 1. The procedure for selecting studies is presented in Figure 1.
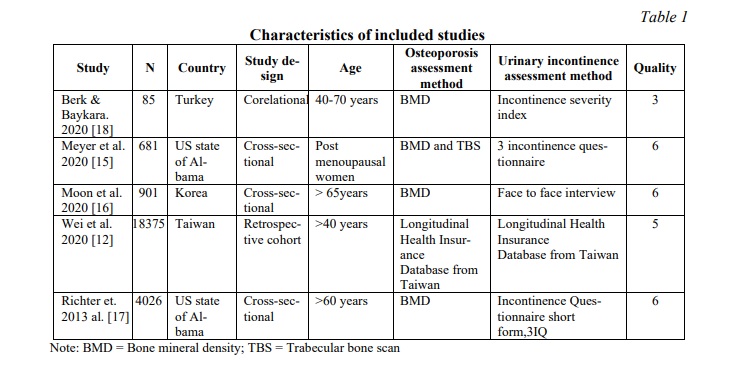
The studies included were carried out in four nations: Turkey, USA, Korea, China (Table 1). Most of the papers [15, 16, 17] were cross-sectional in nature, with one retrospective cohort study [12] and one correlation study [18]. Only women were included in all the studies. A total of 24096 women participated in the various included studies. Majority of the research looked at women between the ages of 40 to 70. All five studies [12, 15-18] (100.0%) described age, body mass index, race, mode of delivery, smoking and drinking status of participants while few also focused on previous health condition, or surgery, physical activity, marital status and previous fractures. The majority of research used questionnaires to track UI prevalence, with only a handful relying on self-reporting (Table 1). Female volunteers were recruited from physiotherapy outpatient departments, universities, physical medicine and rehabilitation outpatient department and general population.
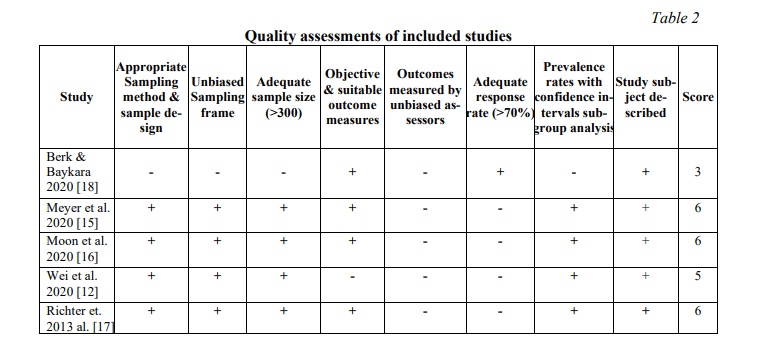
Methodological quality. The quality assessment was done using the Loney et al. method [14], which recommended four moderate and one low-quality studies (Table 2). Disagreements between the three reviewers were addressed through conversation.
Osteoporosis (OP) assessment. There were three different techniques of evaluation used. Bone mineral density (BMD) was utilized to measure OP and osteopenia in these studies. BMD was assessed from the lumbar vertebrae (L1-L4) and the left proximal femur in an anteroposterior position in same patients. When the bone density of the left femur could not be determined, the right femur was examined and lumbar spine was assessed when both femoral lesions could not be quantified. BMD of -1 to -2.5 Standard deviation (S.D.) were classified as having osteopenia, while those with a BMD of -2.5 and below were classified as having OP [15, 17, 18]. Trabecular bone scan (TBS) and fracture risk classification scheme was used in one of the studies by Isuzu Meyer et al. TBS of ≤ 1.31 suggested decreased bone quality, indicating a moderate to high risk of fracture [15].
Urinary incontinence (UI) assessment. Various questionnaires were used to measure urinary incontinence, with some concentrating on the kind of incontinence and others on frequency assessment (Table 2). Isuzu Meyer et al. used the four-item International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) to assess UI [14]. while the three Incontinence Questions (3IQ) were used to determine the kinds of UI among participants [15, 17]. Ejder Berk et al. assessed the frequency and amount of urine loss by another instrument known as Incontinence Severity Index [18] while as the International Consultation on Incontinence Questionnaire was used to assess the frequency and severity of UI by Richter et al. [17]. Face-to-face interview was the method of investigation used to assess UI by Ji Hyun Moon et al [16].
Coexistence of the two conditions. According to a study based on the Korean National Health and Nutrition Examination Survey (KNHANES) data, poor bone health is not alone responsible for UI in elderly females. The lumbar spine adjusted T-score was not statistically significant (p-value = 0.390) in either the UI or no UI groups. Furthermore, neither the entire femur nor the femoral neck T-scores showed statistically significant (p-value = 0.39 and p-value = 0.739, respectively) results [16]. Using the combined quality/quantity bone evaluation, another research found that 262 of 681 individuals had poor bone strength and 419 individuals had normal bone strength. UI (stress, urge, mixed) was more likely in women with poor bone quality i.e. OP or osteopenia [15]. The OP group and the healthy group scores were statistically significant in terms of presence of UI (p-value <0.001) in comparison to osteopenic and healthy group scores (p-value =0.081). Even the OP and osteopenic group scores also showed statistically significant difference (p-value <0.001) [18]. UI risk was 1.79 times greater in women with OP than in women without osteopenia, regardless of age, according to a retrospective cohort analysis. SUI was found to have a stronger relation to OP than other kinds of urinary incontinence [12]. Another study found that women with osteopenia had a decreased risk of UI compared to normal women, but those having OP had a very high frequency of moderate to large volume UI (75.0%) and fecal incontinence (16.0%) [15].
Discussion. The focus of the study was to assess the available research and find links between the coexistence of bone health (OP, osteopenia) and urinary incontinence. The link between OP and UI is clearly of worldwide interest, as evidenced by the research evaluated, which came from different countries of the world. Women of various ages were included, which may have influenced study comparability. Most studies found a strongly significant link between OP and urinary incontinence, whereas one study using KNHANES data found no association. Face-to-face interview was used to assess UI in females, which is not a reliable technique for measuring an outcome, and females are often hesitant to discuss this issue, which can lead to the lack of correlation [16].
Some researches have shown a strong association between osteopenia and urinary incontinence, whereas others have found no link. The majority of the research employed BMD and TBS, which are reliable and established methods for measuring bone mineral density [19, 20]. Four-item ICIQ-SF was used in two studies to assess UI whereas the three Incontinence Questions (3IQ) were used to determine the kinds of UI among participants with urinary incontinence [15, 16]. The Incontinence Severity Index was used to assess the frequency and amount of urine loss [18]. All three questionnaires are reliable to measure types, and other parameters of urinary incontinence [21, 22, 23].
In this study, the five most common associated risk factors were revealed to be menopause, obesity, smoking, physical inactivity, and hyperlipidemia. Those with OP who exercise less are prone to develop UI than the one who are more into exercises.
Limitation. The current study has a number of limitations. We only looked at the studies that were written in English. The occurrence of UI in osteoporotic individuals should be examined in both genders in future research to guarantee that the findings can be applied to both. All of the participants in this research were female. However, this may preclude the findings from being applied to both men and women. A larger sample size would result in more conclusive conclusions. The studies that look at the possible pathophysiological connection between BMD and incontinence should be prioritized. Individual variations such as everyday life situations, living habits, hygiene, and the way people perceive and interpret stresses might all have an impact on urinary incontinence, which cannot be ruled out. Future research with bigger samples and individuals of both genders may contribute more to the literature based on these variables. Not only the kind of urinary incontinence, but also the intensity of the symptoms may have yielded more relevant results if alternative questionnaires had been used. Because of social desirability, many studies employed self-reported measures of urinary incontinence, which leads to a significant amount of bias into such investigations.
This was a descriptive overview of the association between UI and OP, not a statistical meta-analysis. Because the majority of the studies were cross-sectional in nature, no conclusions concerning cause and effect could be drawn. This is something to keep in mind while planning future research.
In contrast, the present study used a systematic strategy that included a published methodology, and adhered to the PRISMA statement. The review reveals the restrictions that are currently in place and identifies the existing limitations of published research into the link between OP and urinary incontinence.
Conclusion. In conclusion, this systematic review investigated the coexistence of OP and UI in women, and it demonstrates that UI (stress, urge, mixed) is more likely to occur in women with poor bone quality because of the same pathophysiology.





















Reference lists