Correlation of Biochemical Abnormalities with the Severity of Hospitalized Covid-19 Patients
Abstract
Background: Coronavirus Disease-19 (COVID-19) has a global impact. The laboratory assessments in Covid-19 illness help in better understanding the disease pathophysiology useful in screening asymptomatic individuals to diagnosis, prognosis and monitoring the affected patients. The aim of the study: To observe the association between biochemical and inflammatory parameters among the hospitalised COVID-19 patients of different clinical severity. Materials and methods: This was a retrospective study carried out with the approval of the Institutional Ethics Committee. The study included patients over 18 years, hospitalised with COVID-19 infection, grouped into three severity groups, admitted to ward, high dependency unit or intensive care unit between May to September 2020. Data collection was carried out by manual perusal of inpatient case sheets, computerised patient data system and transcription database for discharge summaries. The biochemical and inflammatory markers like plasma glucose, renal function tests, serum electrolytes, liver function tests, erythrocyte sedimentation rate (ESR), C-Reactive protein (CRP), d-dimer, ferritin, Lactate dehydrogenase (LDH) at the time of admission were collected. Data was expressed as mean and standard deviation or median and range. ANOVA test followed by post hoc (Tukey) test, Pearson correlation and Receiver Operating Characteristics (ROC) Curve analysis were performed. Results: Significant correlations were observed between the mild and moderate-severe illness groups with respect to fasting plasma glucose, blood urea nitrogen, creatinine, direct bilirubin, total protein, albumin, ferritin and LDH. The AUC was the highest for LDH at 0.64 followed by blood urea nitrogen to creatinine ratio at 0.62. Conclusion: High levels of renal function parameters were potential predictors of acute kidney injury among patients with COVID-19. Fasting plasma glucose, serum albumin, LDH, Blood urea nitrogen (BUN) and BUN-creatinine ratio are better indicators of the severity of the disease with multiorgan dysfunction.
Keywords: COVID-19, SARS-CoV2, acute kidney injury, liver impairment, ferritin, angiotensin converting enzyme 2, electrolyte alteration, lactate dehydrogenase
Introduction. The severe acute respiratory syndrome corona virus 2 (SARS-2), defined as Coronavirus disease 2019 (COVID-19) by the WHO, causes acute respiratory disease. Clinically, almost 80% of the COVID-19 patients are asymptomatic or having mild infection, while 13.8% have severe disease and 6.1% have critical life-threatening disease requiring hospital admission mostly with intensive care support [1, 2]. Individuals over the age of 65 years those with associated co-morbid conditions such as diabetes mellitus, hypertension, Coronary Artery Disease (CAD), Chronic Obstructive Pulmonary Disease (COPD) and obesity are highly susceptible to severe form of the disease. Early screening and identification of these risk factors will avert the chances of developing severe disease, thereby reducing the morbidity and mortality. [3]
Assessment of biochemical alterations induced by the COVID-19 infection could help in better understanding of the disease pathophysiology which could help the clinicians in both assessment and treatment of the affected patients. With surging new cases causing out-stretched health care systems with limited resources, risk stratification becomes crucial in identifying patients requiring intensive care support or hospital admissions.
The aim of the study. Hence this study was conducted to study the biochemical alterations and their association with inflammatory parameters among the hospitalised COVID-19 patients of different clinical severity.
Methods. The index study was a retrospective study carried out at a tertiary care teaching hospital in India. The study population consisted of patients over 18 years of age, hospitalised with COVID-19 infection, confirmed by RT PCR of nasopharyngeal swab. They were admitted to the ward, high dependency unit and intensive care unit between May to September 2020.
Patients were classified into three groups based on the clinical severity as the following:
Group-1: Mild disease -defined as peripheral oxygen saturation >94%
Group-2: Moderate disease -defined as peripheral oxygen saturation between 90-93
Group-3: Severe disease -defined as the peripheral oxygen saturation less than 89%
Data collection was carried out by manual perusal of inpatient case sheets, computerised patient data system and transcription database for discharge summaries. Details of their demographic parameters, presentation symptoms like fever, throat pain, cough, breathing difficulty, anosmia, diarrhoea, myalgia etc, biochemical laboratory test reports such as plasma glucose, renal function tests, serum electrolytes, liver function tests, inflammatory markers like ferritin and Lactate dehydrogenase (LDH) done at the time of admission were collected. The primary outcomes of interest in this study were the clinical severity, elevated inflammatory markers and abnormal biochemical parameters. The study was approved by the Institutional Ethics Committee (IEC-NI/20/AUG/75/49, dated 08-08-2020).
Data were expressed as mean and standard deviation or median and range. An ANOVA test followed by post hoc (Tukey) test was done to compare the variables between the groups and Pearson correlation to obtain the association between the variables. Receiver Operating Characteristics (ROC) Curve analysis was performed to the estimate area under curve (AUC) with 95% confidence interval and cut off point was obtained according to the Youden index. All statistical analyses were conducted using SPSS Software version 16. P value of <0.005 was considered to be statistically significant.
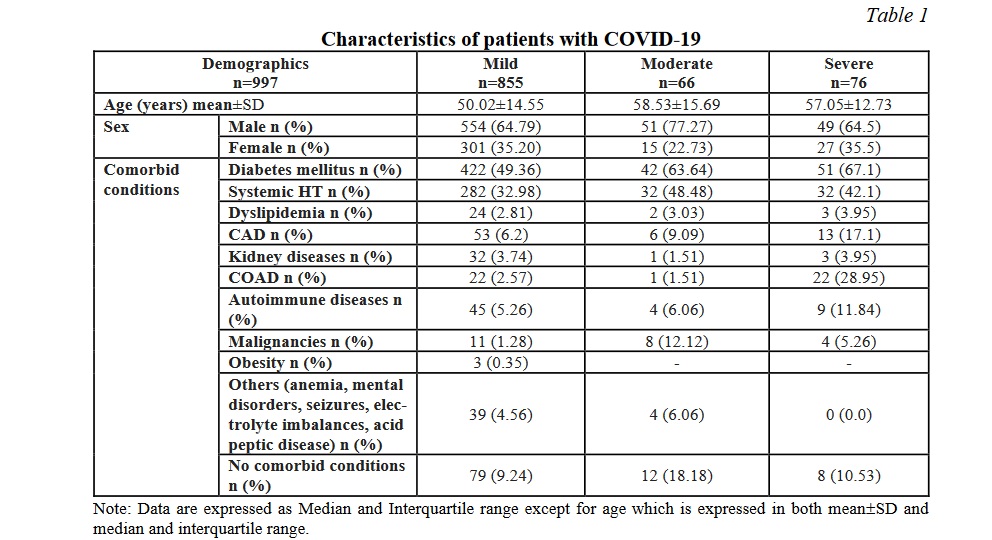
Results. In the present study, a total of 996 patients with confirmed COVID-19 (338 males and 651 females) were included in the analysis of biochemical abnormalities. Out of the 996 included patients, 855 patients (85.89%) had mild, 67 patients (6.73%) had moderate and 74 patients (7.43%) had severe COVID-19 infection. The median age of the patients was 50.02±14.55, 58.53±15.68 and 57.05±12.73 years among the mild, moderate and severe patients respectively. Among the 996 patients, 54% patients had one or more co-morbidities. The most common comorbidities were diabetes mellitus in 487 (48.9%) and systemic hypertension in 345 (34.6%).
The common presenting clinical features were fever, throat pain, cough, dyspnoea, diarrhoea, anosmia, myalgias. Compared to the mild group, both moderate and severe groups were significantly older in their age and were more likely to have underlying medical conditions (62.92% in the mild group vs 86.57% in the moderate group and 86.49% in the severe group). Table 1 compares the baseline characteristics of the patients among the mild, moderate and severe COVID-19 infections. As the glycaemic control worsened the severity of the disease progressed. The severe disease was observed more among the patients with autoimmune diseases, coronary artery disease, kidney disease and chronic airway disorders, while systemic hypertension and malignancies were observed at higher frequencies among the patients with moderate disease.
There were significant differences between the mild and moderate / severe group in the variables such as markers of glycaemic control, inflammatory markers, renal and liver function tests. Tables 2, 3, 4 show the distribution of the biochemical markers among the study participants. Significant correlations were observed between the mild and moderate/ severe illness groups with respect to fasting plasma glucose, blood urea nitrogen, creatinine, sodium, potassium, bicarbonate, urinary protein leak, presence of red blood cells in the urine, direct bilirubin, total protein and albumin. The serum electrolytes sodium, potassium and bicarbonate showed a significant variation as the disease progressed from mild to severe disease. Among them serum bicarbonate showed a very significant variability while the disease progressed from mild to severe, while chloride did not exhibit such variation. Similar findings were noted among the inflammatory markers such as ferritin and LDH. Similarly, the total protein, serum albumin and the albumin-globulin ratio displayed striking significance between the mild and severe disease. Serum ferritin and lactate dehydrogenase showed a very significant difference when compared between the mild and severe diseases. Such a striking variation was also demonstrated by the fasting glucose levels.
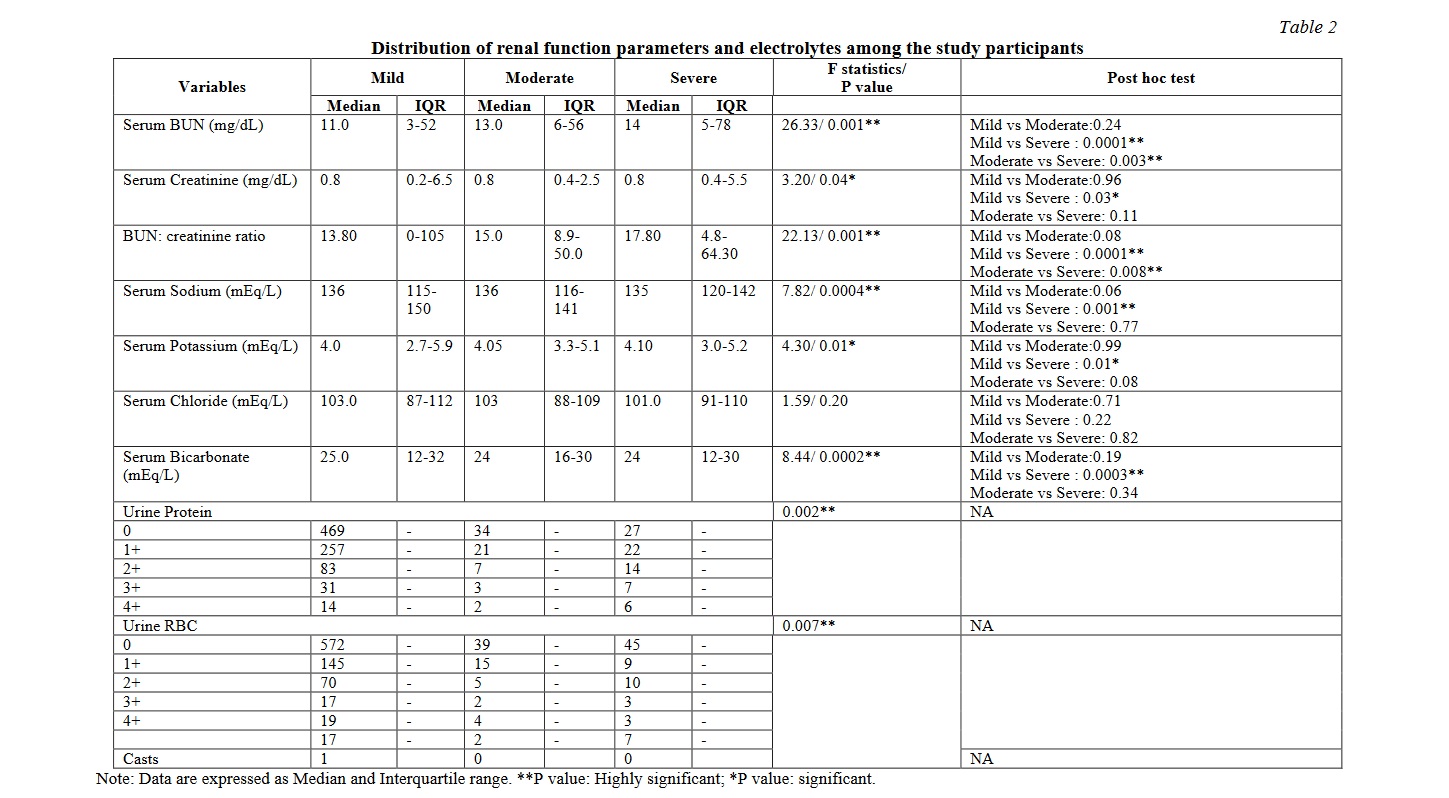
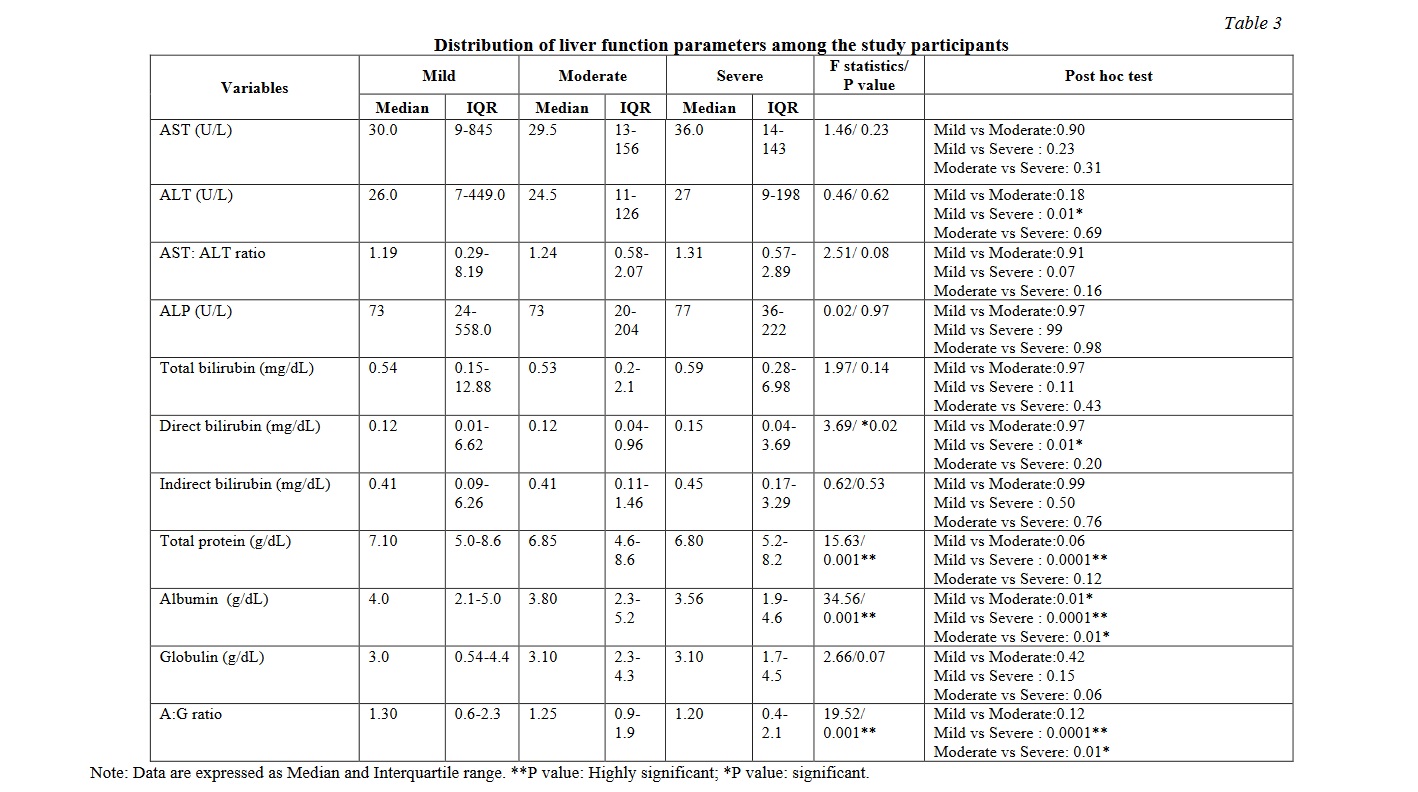
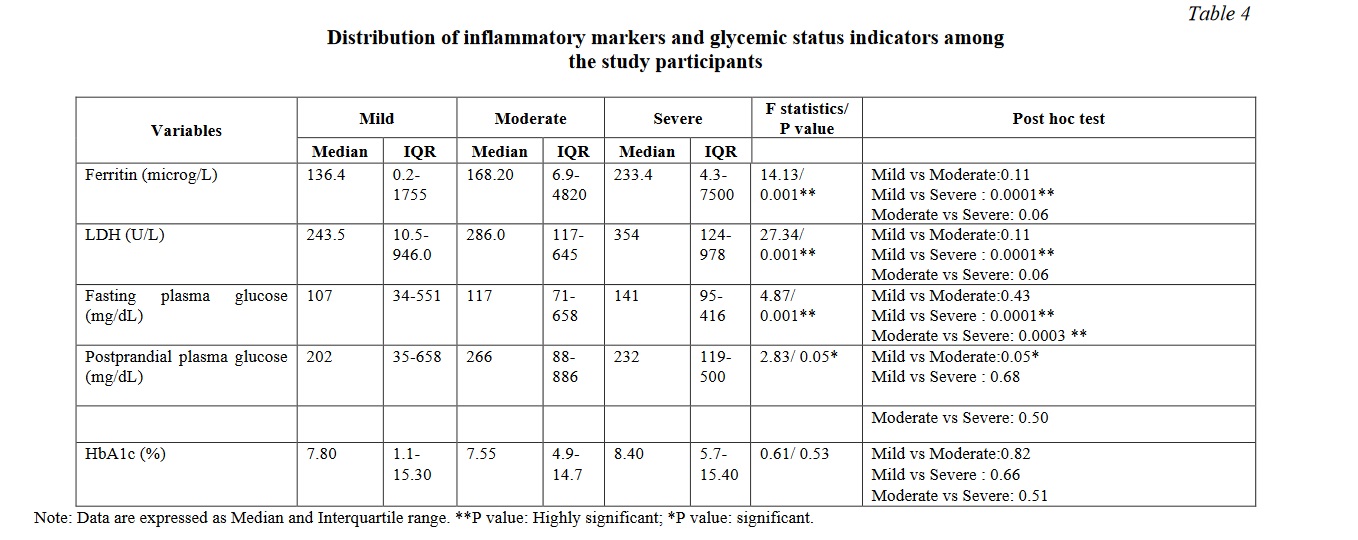
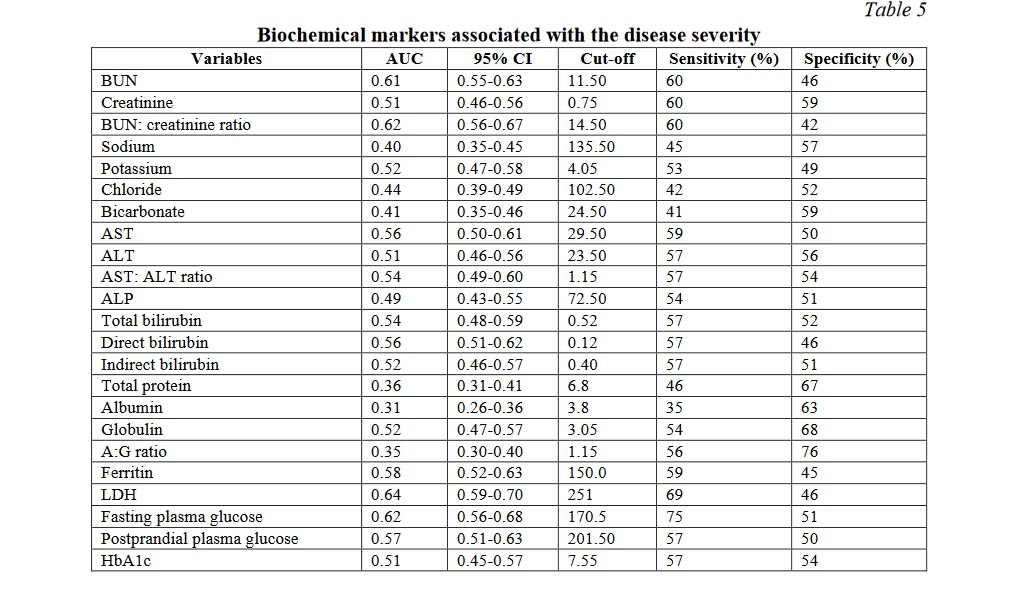
Table 5 demonstrates the area under curve, cut off values along with sensitivity and specificity of the various biochemical markers studied. The AUC was the highest for LDH at 0.64 followed by blood urea nitrogen to creatinine ratio and fasting plasma glucose at 0.62 followed by blood urea nitrogen at 0.61. Fasting plasma glucose had the highest sensitivity of 75% while albumin to globulin ratio had the highest specificity of 76%.
Discussion. COVID-19 has a massive impact globally causing enormous burden on the healthcare system.
The SARS-CoV2 infection is highly contagious and carries a high morbidity and mortality with it [4]. Most of the infected patients suffer from a less severe form of the infection and have a better prognosis [5], however, higher mortality is observed among patients with severe infections. The progress of the disease is highly unpredictable, ranging from asymptomatic or mild to very severe disease. COVID-19 is a multisystem disease affecting almost all organs in the body. Hence the use of biomarkers could help in the diagnosis and management of these patients. An attempt was made to study the common abnormalities noted in the biochemical parameters and their correlation with the disease severity. The patients with moderate and severe illnesses were older and had more than one co-morbid condition than the patients with mild disease. This finding was consistent with previous studies which suggested both age and co-morbid conditions would be strong risk factors for poorer outcomes. [6, 7]
Renal function alterations in the form of elevated blood urea nitrogen (BUN), raised creatinine and increased BUN: creatinine ratios have been reported in previous studies among patients with COVID-19 infection. A significant increase was observed in the index study in BUN, creatinine and BUN: creatinine ratio among the severe cases compared to the mild patients. The likely mechanisms for this could be the kidney tubular cells, that express the ACE2 receptor on their surface get directly infected with SARS-CoV2. The kidney resident cells are affected by circulating inflammatory mediators causing endothelial dysfunction, microcirculatory derangements and tubular injury. Acute kidney injury has been observed among 25-30% of COVID-19 patients and carries higher mortality risk [8, 9].
According to Durvasula et al, the acute kidney injury could be due to the poor inflammatory response and its impact on the renal tubular perfusion [10]. SARS-CoV2 also has a direct cytopathic effect on podocytes and the tubule epithelial cells as the cell entry receptor ACE2 is expressed on them followed by glomerular insult [10, 11]. This explains the progressive elevation noted in blood urea nitrogen and creatinine. In the present study, patients had a steady increase in the BUN as the severity of COVID progressed with P value of 0.001. Within-group comparisons showed that there was significant increase in BUN when mild was compared with severe COVID infections as well as when moderate cases were compared against severe cases. The median value of serum creatinine was almost the same in all the three groups with mild significance due to variation in interquartile range (P=0.04). BUN: creatinine ratio showed significant change across the groups (P=0.001) with the intergroup changes being the same as for BUN. This clearly indicated that patients in the moderate and severe groups could have been in acute kidney injury as indicated by the highly significant alterations in BUN and BUN-creatinine ratio with mild alterations in serum creatinine. (Table 2)
In the present study electrolyte imbalances were observed among severe COVID-19 disease. In the index study significant alterations in the levels of serum sodium, potassium and bicarbonate were observed while comparing the mild and severe cases. Median serum sodium levels were in the lower limit of biological reference interval in the three groups (P=0.0004) and potassium values were normal (P=0.01). There was no significant change in serum chloride values. Serum bicarbonate values were decreased (P=0.0002). Within groups comparisons showed there was a significant difference between mild and severe cases but not between moderate and severe cases. This showed that electrolyte alterations became prominent only when the disease progressed to a severe state (Table 2).
The SARS-CoV2 virus interacts with the ACE2 receptors probably reducing their expression (Fig. 1).
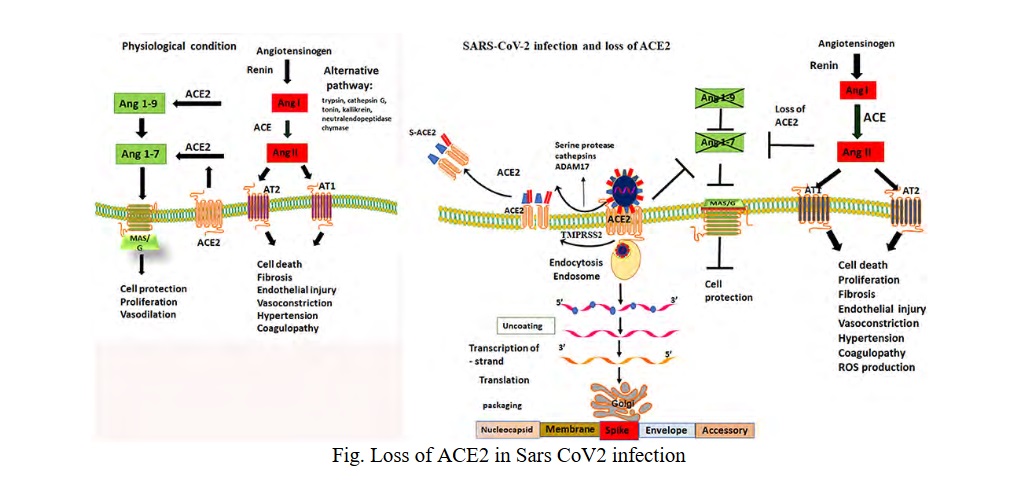
ACE2 is highly expressed in capillary rich organs such as lungs, kidneys, gut and brain. ACE2 regulates the renin-angiotensin-aldosterone system. Normally ACE2 lowers blood pressure by catalyzing the hydrolysis of angiotensin II which is a vasoconstrictor into angiotensin I which is a vasodilator. The pathophysiologic changes that occur as a result of increased angiotensin II [12, 13]:
- Angiotensin II acts on the adrenal cortex to stimulate the release of aldosterone, leading to sodium and water retention and excretion of potassium.
- Binding of Ang II to angiotensin receptors causes vasoconstriction, endothelial injury, endovascular thrombosis and increase blood volume.
- Precipitates hypertension and accelerated thrombosis in arterioles by activating the coagulation cascade (both thrombin and platelets). These thrombogenic effects on the platelets were not reversible with aspirin
- At the cellular level, angiotensin II induces various signalling pathways: serine/threonine kinase, ERK, JNK/MAPK as well as PKC.
- Angiotensin II induces production of IL-6 and TNF-α, possibly through above mentioned pathways.
- Angiotensin II is a potent activator of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; thus it induces production of reactive oxygen species (ROS).
- Angiotensin II activates neutrophils and macrophages flux to the affected tissues and inhibits the production of nitric oxide and hence promotes vascular injury.
Hypokalemia can further be augmented by the gastro-intestinal manifestations of COVID in the form of diarrhoea [14, 15]. The progressive renal insult is also reflected in the steady increase in the urinary protein loss and presence of red blood cells in the urine as the severity of COVID progressed from mild to severe stage (Table 2).
Cholestatic involvement with elevated conjugated bilirubin and cytosis have been observed previously, which could probably be due to the direct toxic effect of the virus on the hepatocytes and bile duct epithelium due to the higher expression of ACE2 receptors in them [16, 17]. But, no significant alterations have been detected in the histopathology of these cells, which raises the possibilities of COVID-19 related liver dysfunction resulting from hepatotoxic drugs, systemic inflammatory response, hypoxia related, multiorgan dysfunction – all causing secondary liver injury [18, 19].
In the present study, there were no statistically significant differences in serum aspartate transaminase (AST), alanine transaminase (ALT), De Ritis ratio, alkaline phosphatase (ALP), total bilirubin and indirect bilirubin. There was a significant difference in direct bilirubin (P=0.023) with significant differences especially between mild and severe groups. Conjugated hyperbilirubinemia was significantly increasing with progressive severity of COVID-19 illness (Table 3).
The present study showed a significant increase in the alanine transaminase (ALT) between mild to severe cases. The De Ritis (AST:ALT) ratio however did not demonstrate a statistically significant difference with progressing severity of the disease. Altered De Ritis ratio was found to have an association in hyperbilirubinemia patients [20]. Studies have shown that more than 50% of the hospitalised patients were found to have varying degree of liver involvement. Though serum transaminases are found to be elevated in COVID-19 patients, their propensity to predict survival or mortality among these patients is unclear. Moderate to severe liver damage is characterised by AST:ALT ratio <1.0 while it is more than 1.0 in severe liver diseases [21]. In the present study, though there was a steady increase in AST:ALT ratio from mild to severe cases, the increase was not statistically significant.
Albumin is largely distributed in intestine, muscle, skin and various body fluids. It is a negative acute phase reactant with low serum levels in acute inflammation and is inversely proportional to the extent of systemic inflammation. Among the COVID-19 patients, an inverse relationship was found between serum albumin levels and the severity of the disease [22]. In the present study, total protein (P=0.0016), serum albumin (P=0.001) and albumin: globulin (AG) ratio (P=0.001) showed statistically significant differences across the groups. When compared within groups total protein showed statistically significant difference between groups mild and severe, whereas albumin showed statistically significant decrease in values between mild and moderate as well as between moderate and severe. And albumin:globulin (AG) ratio also showed statistically significant difference between mild and moderate as well as between moderate and severe. There was no significant change in the globulin values. Thus, serum albumin and AG ratio could serve as good biomarkers for the progression of the disease especially in cases of liver involvement (Table 3).
COVID-19 patients have been found to have elevated muscle injury biomarkers like creatinine kinase, and myoglobin [23]. Such alterations could be a direct effect of the virus on the muscle due to the ACE2 receptor expression or indirect effect as a result of cardiac or renal injury. Significant elevation of lactate dehydrogenase (LDH) was observed in the index study (P=0.001) as observed previously and it progressively increased with worsening severity [24, 25] (Table 4). LDH is a non-specific marker of tissue damage, hence, it emerges as one of the most consistently elevated markers among COVID-19 patients at risk of developing adverse outcomes [26].
Ferritin is an acute phase reactant and it has been well established as a marker for disease progression in COVID-19 [27]. In this study, ferritin showed a steady and significant increase from mild to severe illness (P=0.001). Within-group comparisons showed that both LDH and ferritin showed significant differences only between mild and severe cases and not between moderate and severe cases. Hence LDH and ferritin may not indicate early stage of the disease (Table 4). During any widespread acute inflammation, IL-6 increases the vascular permeability which extravasates the serum albumin into the interstitium causing a drop in its serum level. Moreover, severe COVID-19 patients who were found to have significantly elevated levels of ESR, CRP, ferritin had increased mortality rates. This favours an exaggerated immune response among them [28].
Glycaemic status of the patients was found to have its impact on the COVID severity in the index study. The fasting blood glucose showed a steady increase as the disease severity progressed (P=0.001) and within-group comparisons showed statistically significant differences between mild and moderate as well as between moderate and severe. Hence fasting plasma glucose could serve as a biomarker of the advancement of the disease. Postprandial glucose showed just significance between the groups while glycated hemoglobin did not show significant differences across the groups (Table 4). Data on new onset diabetes from 8 studies with a total patient population of 3711 has shown that a pooled proportion of 14.4% were newly diagnosed [29]. A greater release of hyperglycaemic hormones due to stress induced by the disease leading to increased blood glucose levels appear to play a significant role [30]. The effects of the virus on ACE2 receptor on pancreatic islets and their subsequent changes like direct beta cell damage and unopposed angiotensin II effect which can impede the insulin secretion can explain the glycaemic abnormality in SARS-CoV2 infection [31]. The present study also concurred with this finding as the fasting blood glucose showed a significant trend but the HbA1C did not show such variation among mild to severe covid cases. Fasting plasma glucose, LDH, BUN, and BUN-creatinine ratio are better indicators of the severity of the disease with multiorgan dysfunction (Table 5).
Conclusion. In conclusion, high levels of urea nitrogen, creatinine were potential predictors of Acute Kidney Injury among patients with COVID-19 and also predicted the progression of the disease severity. Similarly, serum albumin, LDH, ferritin and fasting blood glucose potentially predicted disease progression. Electrolyte disturbances in the form of low serum sodium, low potassium and alterations in the bicarbonate levels have been observed among the SARS-C0V2 infected patients, which requires further studies to understand their propensity as morbidity and mortality predictors.
Limitations
It was a retrospective, longitudinal cohort study yet the data were collected from a structured, prospectively maintained database. The exact time of parameter sampling could not be estimated. So, the biomarkers kinetics during the disease progression could not be established. The sample size was small and single centre study and its generalizability across different ethnic groups is unclear.
Thanks
The authors wish to thank the participants who have provided the data for conducting the study and publication of this article. The authors wish to thank the management for providing the necessary infrastructure and support for conducting the study and publication of the article.




















Reference lists