Seasonal periodontal microcirculation by combined stress in rats corrected with complex phytoadaptogen
Abstract
Background: One of the negative inducers of the internal environment is inflammation; amongst external factors the most significant is polyetiological stress. One of the promising pharmacological methods of health protection is based on natural biologically active compounds, especially complex phytoadaptogen (CPhA). The aim of thie study: This study evaluated the seasonal rhythms in periodontal microcirculation under combined stress (CS), and the possibility of seasonal prophylaxis with complex phytoadaptogen – Glycyrrhiza glabra, Rhodiola rosea, Acantopanax senticosus. Materials and methods: The study was carried out on 60 Wistar male rats (230±20 g) kept in natural light. The complex phytoadaptogen is composed from official 70% tincture of Glycyrrhiza glabra and 40% tincture of Rhodiola rosea, Acantopanax senticosus in the ratio 2:1:1. Results: Under seasonal differences in meteorological data in healthy rats systolic flow velocity (S) in winter was higher than in summer (P=0.005); under the effect of CS on the gum there was a seasonal difference of S (P=0.03) with a maximum in winter (P=0.007); decrease in both seasons of the Gosling index (PI) (winter (P=0.008), summer (P=0.005)), peripheral vascular resistance (RI) (summer (P=0.005), winter (P=0.01)), Stewart index (SD) (summer (P=0.005), winter (P=0.01)). Under administration of CPhA (groups 5 and 6) the microcirculation indicators in the gum and peripheries were statistically significantly different from those in experimental periodontitis, and there were fluctuations within the confidence interval of the norm (p<0.01), seasonal differences RI (P=0.005) and SD (P=0.03), there was a significant leveling of the damaging effect of CS. Conclusion: Application of a novel herbal extract inhibited alveolar bone resorption by the reduction of osteoclastic activity, maintained the integrity of periodontal structures, and normalized the periodontal microcirculation in summer and winter.
Keywords: combined stress, complex phytoadaptogens, immobilization stress, experimental periodontitis, microcirculation, seasonal rhythms
Introduction. Features of seasonal cycles of mammals are formed depending on the ambient photoperiodism, temperature, and are characterized by a number of differences in the production of melatonin and its derivatives, and the restructuring of the neuroendocrine immune system, which, controls seasonal reorganization of body systems through the central circannual pacemaker system and may depend on epigenetic factors [1]. The duration of the daytime cycle is the main regulator of biorhythmological changes in the cardiovascular system and human behavior. Seasonal changes in physiological functions are determined by melatonin regulation of TSHß expression in pars tuberalis and hypothalamic dio2 (dio2 gene expression) [2], and are reflected in neurotransmitters. Seasonal changes in the regulation and functioning of macrohemodynamics have been studied thoroughly [3, 4]; however, there are few studies evaluating the characteristics of seasonal changes in microcirculation in normal conditions and in various typical pathological processes.
Annual cycles are also typical for adaptive responses and the immunity. Analysis of mRNA expression levels in the peripheral blood and adipose tissue monocytes from different ethnic groups showed that about 23% of the genome had significant seasonal differences, with two different antiphase patterns, when the expression of one set of genes is increased in the summer and the expression of the other in approximately equal parts is increased in the winter [5]. Pars tuberalis plays an important modulating role in the formation of the seasonal response to stress and immune functions, which may be important in the pathogenesis of inflammation [6]. Some studies showed a significant decrease in the functional activity of immunocompetent cells in the winter [7] associated with increased energy expenditure which was considered as the result of immune response [8].
Receptors for neurotransmitters on the membrane of lymphoid cells providing perception of changes in the neurotransmitter microenvironment by immunocytes have been discovered. The idea of an open synapse linked these components into an integral chain that provides the possibility of interaction between the nervous and immune systems through neurotransmitters. It was shown that endogenous bioregulators, including glucocorticoid hormones and immunomodulating cytokines, in particular, interleukin-1 (IL-1), the first of discovered and characterized interleukins, which initiates a cascade of innate and acquired defense reactions, play a decisive role in these mechanisms. Thus, seasonal changes in the NEIM system determine the seasonal variability in the activity of adaptive processes, inflammation, and other typical pathological processes [9].
Biologically active substances of phytoadaptogens (PhA), or modifiers of the biological response, affect central and local regulatory factors, modulate the state of the central nervous and endocrine systems, and the sensitivity of cellular receptors to the action of neurotransmitters and hormones [10-14]. Phytoadaptogens are used as a part of long-term therapeutic and preventive therapy, since they rarely cause side effects. CPhA have individual therapeutic activity and tolerance increasing with time, so their combined compositions are recommended. Among the well-known and widely used phytoadaptogens there are Glycyrrhiza glabra, Rhodiola rosea, Acanthopanax senticosus and many others [14-18].
The choice of the studied drugs is based on the chemical composition, pharmacophores, and targets of Glycyrrhiza glabra, Rhodiola rosea, Acanthopanax senticosus. Glycyrrhiza glabra contains up to 24% of triterpene saponin glycyrrhizin, which causes pronounced anti-inflammatory activity with inhibition of exudative and proliferative phases of inflammation. Glycyrrhizin of Glycyrrhiza glabra significantly reduces the secretion of necrosis factor-α (TNF-α), IL-1β and IL-6; inhibits RANKL-induced osteoclastogenesis. Acanthopanax senticosus contains antioxidants – pinocembrine, glabranin, which reduce the production of nitric oxide and cortisol in stress. Rhodiola rosea contains polyphenols such as flavonoids, proanthocyanidins, tyrosol, cinnamon alcohol, glycosides, organic acids, essential oils, sugars, fats, alcohols and proteins [10, 18]. The pharmacological effects of Rhodiola rosea: stimulate the central nervous system, increase the level of beta-endorphin in the brain. Beta-endorphin is the stress-relieving, feel-good, analgesic peptide. The main effects of Rhodiola rosea described are adaptogenic, that means “natural herbal products that are nontoxic in normal doses produce a nonspecific response and have a normalizing physiologic influence, stress protective. Moreover, it has been described as an antioxidant [17, 18]. The above effects of Glycyrrhiza glabra, Rhodiola rosea, Acanthopanax senticosus can have a positive effect in the prevention of microcirculation parameters in the periodontium under combined stress (CS) – experimental periodontitis (local factor) and immobilization stress (IS) (centralized exposure).
The aim of the study. To evaluate the seasonal rhythms in periodontal microcirculation under combined stress (CS), and the possibility of seasonal prophylaxis with complex phytoadaptogen – Glycyrrhiza glabra, Rhodiola rosea, Acantopanax senticosus.
Materials and methods. The experiment was performed on 60 adult Wistar male rats (obtained from laboratory animal nursery “Rappolovo”), weighing 230±20 g. The animals were kept in cages (5 animals in each) in natural light (43°01'00"N 44°41'00"E, Vladikavkaz) with free access to food and water. Food was given once a day (from 9.00 to 10.00 o'clock a.m.). The rats were kept in a room with controlled temperature (21±1ºC) and humidity (50-55%) and in natural light.
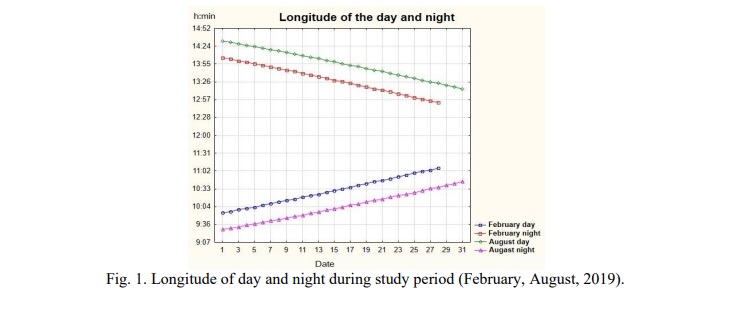
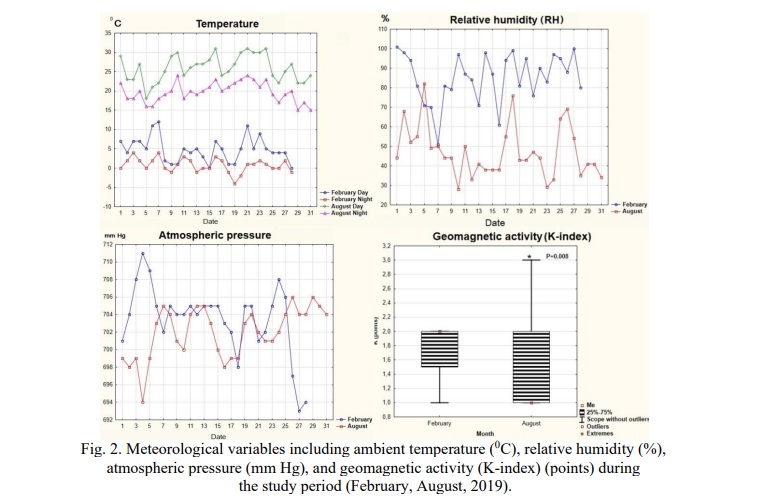
The study used 2 time periods – winter (February) and summer (August), the only difference that affected the condition of animals was the value of the photoperiod (depending on the season). All meteorological data recorded in this study are shown in Figure 1,2. All efforts were made to minimize animal suffering, to reduce the number of animals used.
After the first adaptation period, the animals were randomly assigned to the following groups – 10 animals in each: winter/summer control (1-2 groups), combined winter/summer stress (3-4 groups) and winter/summer combined stress under administration of complex phytoadaptogens (5-6 groups). All the operations were performed between 09.00 and 12.00 PM (GMT).
Combined stress in the summer and winter was modeled by a combination of experimental periodontitis (local factor) and immobilization stress (IS) (centralized exposure).
The study was approved by the Ethics Committee of the Institute of Biomedical Investigations – the Affiliate of Vladikavkaz Scientific Centre of Russian Academy of Sciences (Protocol 6, January 24, 2019).
Experimental model of combined stress (CS) can be comparable in pathogenesis to human stress, and is implemented with the participation of local and general etiological factors. The local factor is a metal ligature that disorders the integrity of the ameloblastic epithelium ("hydrostatic cushion") of the lower incisors of rats, which excludes the restoration of periodontal tissues by its cells and contributes to the maintenance of alterations. A common factor – a high-carbohydrate diet and IS, contribute to the generalization of the inflammatory response [19].
The local factor in CS is experimental periodontitis (EP). EP was modeled by applying a metal ligature at the gingival margin of the lower incisions, using "Zoletil" as an analgesic at a dosage of 0.1 ml/100g. The ligature was fixed to the crest of the alveolar process with a silk thread, disturbing the integrity of the ameloblastic epithelium (hydrostatic cushion) on the vestibular side, where it has the smallest thickness in order to create an entrance for microflora. The animals were fed with wheat porridge with milk (30%), starch (20%) and sugar (15%) (High-carbohydrate diet according to A. I. Evdokimov) [19]. The intensity of inflammation in the periodontium depends on the reactivity of the body and on the effects of external factors, but together with the ligature, they can contribute to the development of a pronounced inflammatory process [20]. This model causes capillary stasis in the microcirculatory system due to perivascular tissue edema in inflammation.
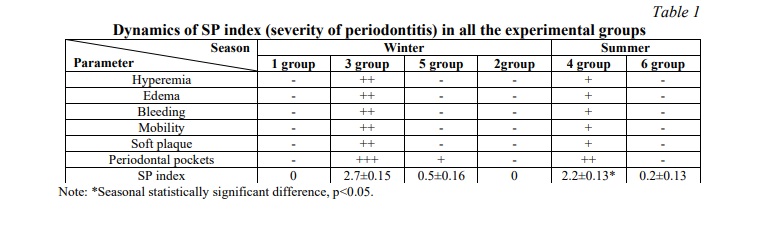
To assess the severity of the inflammatory process in the rat periodontium and intersystem correlation analysis, we developed an index reflecting the severity of peritonitis (SP). Evaluation scale: 0 – intact periodontal (gums are pale pink, do not bleed); 1 – gums are pale pink, when probing bleeds, periodontal pockets are not detected; 2 – gums are hyperemic, edematous, loose, bleed when probed, periodontal pockets up to 1.5 mm deep, mobility of the first degree; 3 – gums are hyperemic, edematous, profusely bleed when probed, periodontal pockets depth up to 3 mm, mobility of the II degree.
In our experiment, the common factor – IS was modeled by placing animals from day 2 to day 7 in a tight plastic cage with an area of 0.005 m3 for 6 hours from 900 to 1400 [19], totally 6 days.
Small pieces of gum tissue (including the free gingiva, the interdental papilla and the attached gingiva) were fixed in 10% buffered formalin for histopathological studies. The fixed tissues were washed in running tap water, dehydrated in acetone, cleared in benzene, and immersed in paraffin wax (melting temperature 60-62°C). Paraffin sections were cut at the thickness of 4-5 µ and stained with Lily Mayer’s hematoxylin and 2% water soluble eosin. The study was carried out using a polarizing microscope with a digital camera ZEISS Axio Lab.A1 (Germany). In gum tissue the following histological parameters were evaluated: 1) intensity of the inflammatory process; 2) structural pattern of the connective tissue.
Doppler ultrasound was used to access microcirculation (MC) disorders (Angiodin-PC device, Russia, 16MHz probe). For this task each rat was fixed on a wooden board in the supine position, upper and lower jaws were anchored in an open position. In the attached gingiva of the transition fold near the lower incisors, an area where large blood vessels do not pass was selected to examine the liquid exchange in the tissues – systolic (S), diastolic (D), medium (M) blood flow velocity, PI – pulsation index (index Gosling), RI – peripheral resistance index (index Purcell), SD – systolic-diastolic index (Stewart index).
CPhA is composed from official 70% tincture of Glycyrrhiza glabra and 40% tincture of Rhodiola rosea, Acantopanax senticosus in the ratio 2:1:1 [19]. The dose was calculated taking into account the average daily volume of liquid consumed and the coefficient (x10) for small laboratory animals (0.1 ml/100g) per day. CPhA was administered with drinking water in a therapeutic and preventive mode (groups 5 and 6) – 14 days before the experimental simulation and 14 days throughout the simulation.
Since the CPhA contains alcohol extracts of PhA, additional control groups of rats were formed. 1,6 % ethanol solution was administered to these animals (the concentration of ethyl alcohol in the applied dose of CPhA) in the therapeutic and preventive regimen. In both seasons, an additional control group was formed taking the anesthetic Zoletil. No significant changes were detected, so we combined the animals into common groups.
Data analysis was performed using Statistica 10.0 software («StatSoft, Inc», Russia). The normality distribution of continuous variables was tested with the Shapiro-Wilk test. The Kruskal-Wallis test was used to compare independent groups of variables with a non-normal distribution. Median (25-75 ‰) values were given as descriptive statistics due to the small number of variants in the sample (the Mann-Whitney-Wilcoxon test). The correlation analysis was performed using the Spearman method. The value of p < 0.05 was accepted as statistically significant.
Results
Inflammatory process
The indicators of the periodontitis severity index are given in Table 1.
Histological examination of gum tissue
On the 1st day of the experiment (control) in animals of groups 1 (winter) and 2 (summer), the gum was pale pink, did not bleed during probing, and pathological mobility of teeth was not revealed. Under histological examination the gum was represented by its own plate of the mucous membrane, covered with stratified squamous keratinizing epithelium. The lamina propria of the mucous membrane consisted of relatively thin collagen fibers arranged in an orderly manner with low-activity fibrocytes lying between them.
Group 4 animals (summer animals) were diagnosed with signs of inflammation: oedema and destruction of collagen fibers, the formation of highly vascularized granulation tissue, with the presence of histiocytes, lymphocytes and plasma cells (Fig. 3a.I) and areas of destruction of the stroma of the gingival mucosa (Fig. 3a.II) at 100%. In group 3 (winter), more pronounced activity of the inflammatory reaction was diagnosed in comparison with animals of group 4 (summer), extensive areas of necrosis of the collagen stroma of the mucosal lamina propria (Fig. 3b.I), an increase in the number of histiocytes, lymphocytes and plasma cells (Fig. 3b.II).
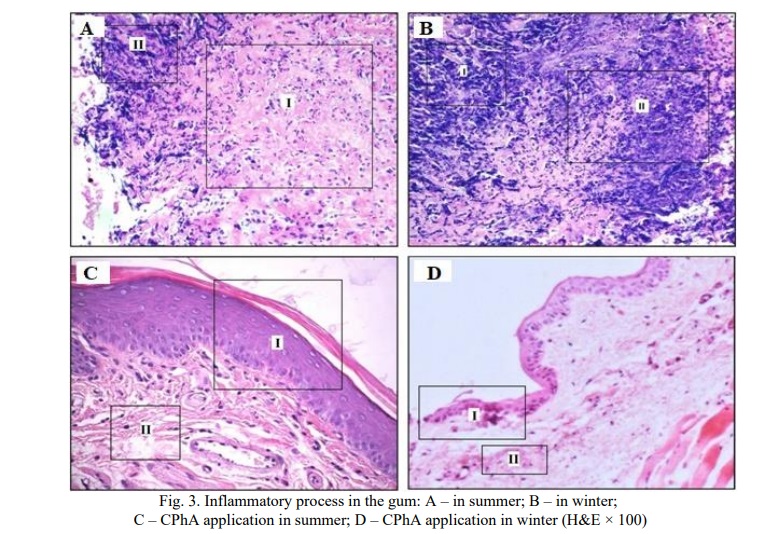
In group 5, rounded cavities were found in the gaps between the bundles of collagen fibers, the vessels were moderately dilated, the inflammatory infiltrate was completely resorbed, and the processes of fibrillogenesis were active. Collagen fibers had a normal structure, but in comparison with intact periodontal preparations they were deformed and thickened (Fig. 3d.II). The stratified squamous keratinized epithelium was not completely restored (Fig. 3d.I). According to clinical and histological criteria, complete resolution of the inflammatory process was achieved only in 6 group by the end of treatment, the stratified squamous keratinized epithelium (Fig. 3C.I) and collagen fibers had a normal histological structure (Fig. 3C.II).
Microcirculation
In healthy animals, there was a statistically significant maximum of the systolic component of blood flow on the gums in winter, which reflects the dominance of circadian regulation. In conditions of CS on the gum and periphery, there was a statistically significant increase in all blood flow velocities in both seasons (groups 3 and 4), seasonal differences were significant for the systolic velocity and (P3-4=0.03) on the gum with a maximum in the winter season (P1=0.007), and with a minimum in the summer (P2=0.01), systemic hyperperfusion developed, more pronounced in winter (Fig. 4). There was a significant systemic decrease of vascular resistance to the blood flow-Gosling index in both seasons (winter (P=0.008) and summer (P=0.005)), peripheral vascular resistance (summer (P=0.005) and winter (P=0.01)), the Stewart index (summer (P=0.005) and winter (P=0.01)). Under CPhA correction of microcirculation parameters of groups 5 and 6 in the winter season there was an increase in the Gosling pulsatory index (0.005) and the Stewart index (P=0.03).
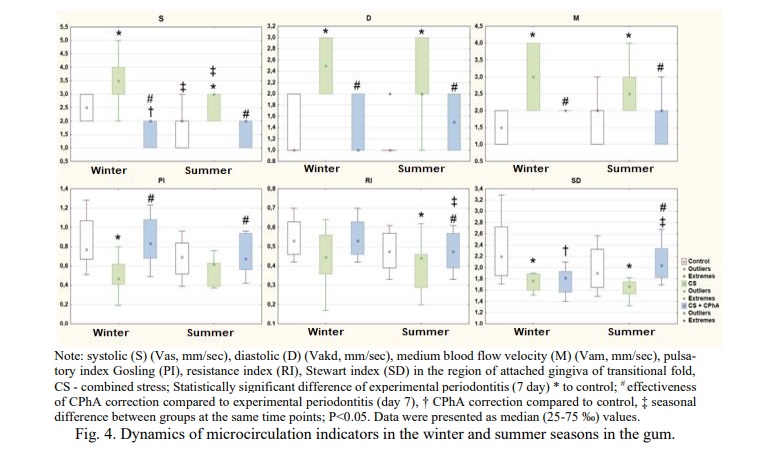
Under administration of CPhA (groups 5 and 6) the microcirculation indicators in the gum and peripheries were statistically significantly different from those in experimental periodontitis, and there were fluctuations within the confidence interval of the norm (p<0.01), seasonal differences RI (P5-6=0.005) and SD (P5-6=0.03) (Fig. 4,5), there was a significant leveling of the damaging effect of CS.
Local inflammation in winter occurred at the maximum of systolic and diastolic components, statistically significant hyperperfusion (increased M) developed with a decrease in permeability and peripheral resistance, which contributed to alteration and was confirmed by histology, consistent with data on a decrease in immunoreactivity and a more severe damage to the rhythmic organization in the winter season. At the same time, a similar hyperperfusion was observed on the periphery in winter. In summer, peripheral MC disorders occurred as a tendency to an increase in the systolic and diastolic components of blood flow, and a decrease in PI and RI.
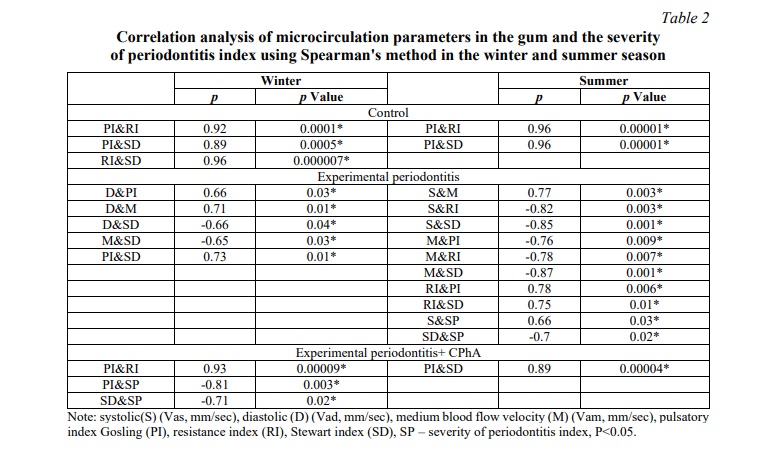
In normal conditions (groups 1 and 2), correlations were noted at the level of vascular parameters (PI, RI, SD). Under EP (groups 3 and 4), the main component of regulation in winter was the metabolic component (D), and in summer – the circadian component, through the systolic component of blood flow. Under EP correction with CPhA in winter the degree of inflammation activity (SP) correlates with local blood flow disorders. In summer, there was a partial restoration of correlations (Table 2).
Discussion. In the study, we obtained statistically significanct data on the presence of seasonal dynamics: in healthy animals – in systolic regulation of blood flow (S) with a maximum in the winter season, which correlates with previous studies [21, 22]; in CS, the degree of hyperperfusion is more pronounced in winter, when all structural components of the periodontium are involved in inflammation with maximum manifestations of structural and tissue damage and the appearance of grade II mobility; CPhA normalizes the state of microcirculation and the morphological status of the periodontium in both seasons, the effect is most pronounced in the summer season.
Seasonal dynamics of microcirculation in the gingival region in both the winter and summer seasons may be associated with the antioxidant effect of melatonin and its increased secretion in the winter season. Several studies have shown that melatonin, which acts as an antioxidant, has a significant protective potential against oxidative stress-induced inhibition of osteoblast differentiation inhuman mesenchymal stem cells (MSK). Melatonin also reduces oxidative stress-inhibited osteogenesis by restoring in vitro differentiation potential of human MSCs through the activation of AMPK-FOXO3a-RUNX2 axis (FOXO3a (the key transcription factor regulating oxidative stress-induced cellular response) and RUNX2 (the key transcriptional factor initiating osteogenesis) protein levels during human MSC osteogenesis.
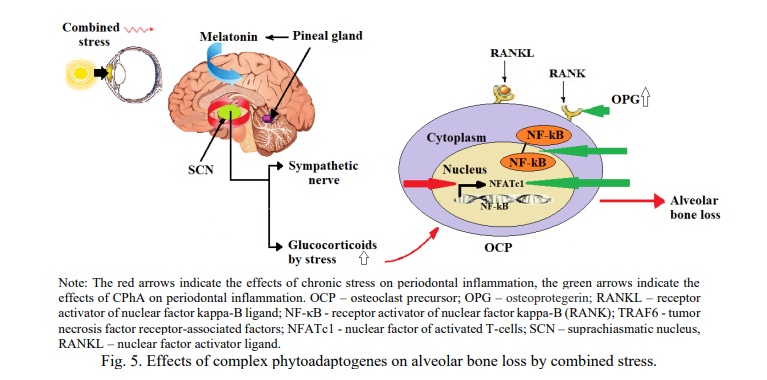
Effect of complex phytoadaptogen on alveolar bone loss by combined stress.
Glucocorticoids are the most important factors in the transmission of circadian time from suprachiasmatic nucleus (SCN) to peripheral osteoclasts (Fig. 5), and it is the peripheral clock of osteoclasts that can regulate the circadian rhythm of bone resorption by regulating the expression of cathepsin K (CTSK) and the nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1). These mechanisms explain the more pronounced mobility of the lower incisors under the effect of combined stress in the winter season (II degree of mobility of the lower incisors, the SP index was 3 points in group 3).
Stress can affect wound healing (pathophysiological effects of changes in cellular mediators). Intracellular signaling mediators Smad3 and Snail also show circadian expression, while circadian rhythms have been shown in human gingival fibroblasts (HGF-1) and mesenchymal stem cells (MSC), and brain-musclearnt-like-protein 2 (BMAL2), vascular endothelial growth factor (VEGF), and PERIOD 3 (PER3) show circadian expression in human MSC [23].
CPhA have an effect on combined stress in several ways (Fig. 5): glycyrrhizin of Glycyrrhiza glabra significantly reduces the secretion of necrosis factor-α (TNF-α), IL-1β and IL-6; glycyrrhizin significantly inhibits RANKL-induced osteoclastogenesis, regulates the expression of nuclear factor of activated T cells 1 (NFATc1); glycyrrhizin reduces the formation of reactive oxygen species in osteoclasts by inducing AMPK (AMP-activated protein kinase) phosphorylation and nuclear transfer of NRF2 (nuclear factor-erythroid 2-related factor 2) [15].
Adaptogens also contain antioxidants: pinocembrine, glabranin, and licorice (licorice) [14], reducing the production of nitric oxide and cortisol in stress [24]. An adaptogen targets mediators of extracellular communication, intracellular networks, and signaling pathways that are involved in stress-induced disorders such as inflammation, atherosclerosis, metabolic disorders, intoxication, and others [25].
Secondary metabolites of adaptogens adapt cells to stress, which is called the phenomenon of hermesis or preconditioning (ashormesisor pre-conditioning) [25, 26]; under the effect of Fapri, the transcription factors NF-KB and FOXO, neurons adapt to stress [27], which plays a role in the adaptation of the NEIM system to the photoperiod.
Seasonal effects of adaptogens on CS are formed through the epiphysis with the participation of melatonin and its receptors. Melatonin has an anti-stress effect, changing the biochemical and neurochemical processes in the hypothalamic structures of the brain [10].
An important feature of the study is the conditions that are as close to natural as possible, in which NEIM-the restructuring of ultra, circa-and infradian rhythms of the body's functional systems is preserved when the seasons change, and the artificial imposition of rhythm destabilizes the temporary organization and does not reflect adaptation to geomagnetic and climatic conditions of the environment. Thus, the significant difference (P<0.01) in day time and night time temperature, humidity, atmospheric pressure, geomagnetic activity (with a maximum in winter, but a maximum amplitude in summer) affects the adaptation of the cardiovascular system (Fig. 1). Studies in natural seasons year-round time (circannual time keeping) allow to work with its main features – its predictive ability to anticipate and prepare for upcoming seasonal changes in the environment [21] and also to express stable annual cycles which at certain phases overlap the effects of the nearest signals, including photoperiod, and artificial modulation of the photoperiod disables these mechanisms. In healthy individuals, tissue functions under the influence of a mechanism that generates endogenous rhythms are reprogrammed between the subjective perception of winter and summer, and a disturbance of the natural seasonal rhythm leads to disorders of adaptive responses [5].
CS leads to the permissive effect of blocking melatonin receptors with glucocorticoids, while CPhA reduces the concentration of stress hormones and turns off blocking of melatonin receptors in peripheral vessels, restoring the physiological properties of melatonin [28]. But it does not explain why the degree of damage is higher under conditions of higher melatonin concentration (winter), and the effects of CPhA are less pronounced. This is the subject of our further research.
The study of seasonal features of microcirculation disorders is necessary for understanding the pathogenesis of post-stress disorders in the dynamics of the annual cycle, developing principles of prevention and correction, taking into account the annual restructuring of the NEIM system.
Conclusions. In healthy animals, there are seasonal differences between parameters of microcirculation. Combined stress caused by a combination of immobilization and local inflammation causes significant changes in hemodynamics, structural tissue damage in both seasons of the year, but the degree of the damage is higher in winter compared with summer. Application of a novel herbal extract inhibited alveolar bone resorption by the reduction of osteoclastic activity, maintained the integrity of periodontal structures, normalize the periodontal microcirculation in summer and winter. These results suggest that complex phytoadaptogen have ameliorative effects on the progress of periodontal breakdown and might be utilized for the treatment or the prevention of periodontal diseases.
Financial support
The research was funded within the framework of the comprehensive topic of the state assignment for the research work of the IBMI VNC RAS (2019-2021).





















Reference lists