Assessment of malnutrition risk in post-stroke patients: a systematic literature review
Abstract
Background: Stroke is one of the main causes of death, especially when associated with malnutrition. Assessment of nutritional status in all stages of the disease is therefore mandatory to improve clinical outcomes. The aim of the study: To identify the most suitable nutritional screening tools in the acute and chronic phase of the disease. Materials and methods: A systematic literature search was conducted in the PubMed, Embase, Cinahl, Scopus and Web of Science databases, and through manual search of relevant journals and grey literature. The process of screening, selection and inclusion of the articles, as well as the assessment of risk of bias and methodological quality, were conducted independently by two reviewers. Out of 1,722 records identified, 20 studies were included in this systematic review. Results: In the acute phase of stroke, the Malnutrition Universal Screening Tool has shown a greater capacity to identify malnutrition. It also correlates better with poor clinical outcomes, such as length of hospital stay, mortality, length of stay and functional disability, when compared to other tools. In the rehabilitation/home setting the Mini Nutritional Assessment demonstrated high sensitivity and predictiveness and strong correlation with clinical outcomes such as quality of life, functional outcomes and Activities of Daily Living. Conclusion: The nutritional status of post-stroke patients is often compromised, and malnutrition is a frequent complication. Identifying specific nutritional screening tools applicable during different stages of the disease helps to better identify the risk of malnutrition, improving clinical outcomes.
Keywords: stroke, nutritional assessment, nutritional screening tool, malnutrition, systematic review
Introduction. Strokes are one of the most common acute neurological diseases and a leading worldwide cause of mortality and physical disability in adults [1]. In Italy, about 90,000 strokes occur every year, about 80% of which are new episodes [2]. Currently in the European Union (EU), there are about 6 million stroke survivors [3]. According to the World Health Organization, the number of stroke events in these countries will increase from 1.1 million in 2000 to more than 1.5 million per year in 2025 due to demographic changes [4]. The risk of stroke rises with age, hypertension, cigarette smoking, heart disease, diabetes, transient ischemic attacks, lack of exercise, alcohol abuse, poor diet and obesity [5].
Stroke is the cause of 10-12% of all deaths per year. It represents the first cause of disability and the second cause of dementia and neurological deficits with loss of self-sufficiency and development of complications. Among these neurological deficits, dysphagia represents a frequent complication [6], with a prevalence between 31.7% and 59.6% of subjects affected by ischemic-hemorrhagic stroke [7], exposing them to morbidities such as dehydration and aspiration pneumonia, and leading to prolonged hospitalization and increased mortality rates [6, 7, 8]. Right from the start, dysphagia also has a notable impact on the patient's nutritional status [9, 10], hampering adequate daily nutritional intake [11] and causing the onset of a state of malnutrition [12, 13], hindering survival [1, 14] and functional recovery [10].
Increased awareness among healthcare professionals and proper management of nutritional issues during and after hospitalization following a stroke can contribute to better outcomes [15, 16]. Early identification and treatment of patients at risk for malnutrition is important [10]. Hence an adequate nutritional screening tool needs to be used with this population, both in the acute and chronic phases of the disease [3]. Numerous screening tests are available [17], but to date only the Malnutrition Universal Screening Tool (MUST) is recognized as a validated tool for patients with acute stroke [3, 18].
The main guidelines drawn up by international scientific societies recommend that every patient suffering from an acute cerebrovascular event should be screened for malnutrition within 48 hours of admission to hospital [3, 18-22]. However, guidelines don’t specify which tool should be used in stroke patients.
In particular, according to the guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN) and of the National Institute for Health and Care Excellence (NICE), the MUST scale is suitable and valid for patients in the acute phase of the disease [3, 18]. The Norwegian National Guidelines [18] for the screening of malnutrition and acute stroke dysphagia consider the Nutrition Risk Score 2002 (NRS 2002) as a suitable tool for use in Norwegian hospitals. The Slovenian Guidelines [19] for nutritional support of stroke, as well as the Canadian recommendations [20] for good practice in hospitalization, inpatient and home rehabilitation in stroke, indicate the importance of nutritional risk assessment without specifying which tool should be preferred among MUST, NRS 2002, Mini Nutrition Assessment (MNA) or Short Nutritional Assessment Questionnaire (SNAQ) [20]. Finally, the Guidelines of the German Society for Clinical Nutrition (DGEM) [21] identify the NRS 2002 as the most suitable screening tool for acute stroke patients, but indicate that other screening and evaluation tools such as MUST, MNA, Mini Nutrition Assessment Short-Form (MNA-SF), may be applicable to other contexts such as rehabilitation and home care.
Some reviews of the literature have been published to date. The reviews by Ray et al. [22] and Sabbouh et al. [23] emphasized the importance of assessing nutritional status in stroke patients, but considering them only in the acute phase of the disease. Both reviews identified the MUST as a possible tool to apply. The review by Wang et al. [24], which considered the correlation between assessment of nutritional status measured by different screening tools and various risk factors and conditions associated with this pathology, hypothesized that both NRS 2002 and MUST could be used to screen for the risk of malnutrition in stroke patients, while MNA was appropriate for people over the age of 65 with associated stroke. In addition, as suggested by the aforementioned reviews [22, 23, 24], the assessment of dysphagia should always be associated with the assessment of nutritional status [4, 25].
To date, it is unclear which specific reference tool for the assessment of nutritional risk is applicable to stroke patients.
Systematic Review objectives. The purpose of this systematic review is to identify through the analysis of current data which malnutrition risk assessment tools may be used in stroke patients, and in which phase of the disease (acute or chronic) and treatment setting (hospital stay, rehabilitation or home) they are applicable. Secondary outcomes will evaluate the identification of malnutrition prevalence and its correlation with relevant clinical outcomes such as: mortality, length of stay, discharge outcomes, functional capacity and quality of life.
Methods. As a preliminary step, relevant guidelines published by recognized scientific societies were identified [1, 10-14]. A systematic and comprehensive literature search was then conducted first in the Cochrane Library and subsequently in the Pubmed, Embase, Cinahl, Trip, Scopus and Web of Science databases. A manual search was also conducted in relevant journals, and by screening the references of relevant articles, and in sources of grey literature such as Google Scholar search engine.
The screening, selection and inclusion process of the articles was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Supplementary file 1) [26, 27]. The risk of bias and the methodological quality of the articles included were independently assessed by two reviewers (MS, SM) through the critical appraisal tools for Analytical Cross-Sectional Studies and Cohort Studies [28] of the Joanna Briggs Institute (Data availability statement).
The search was carried out using the keywords "stroke", "nutritional assessment", and "nutritional screening tool", suitably combined with Boolean operators in search strings adapted to the specificities of the different databases, for literature published between 01-01-2012 and 07-04-2022; full search strategies are available online in data availability statement at the link provided within data availability statement.
The inclusion criteria considered patients with both acute and chronic stroke, aged ≥ 18 years, using at least one validated nutritional screening and assessing both the ability of the screening tool to identify malnutrition and its correlation with relevant clinical outcomes such as: mortality, length of stay, outcomes at discharge, functional capacity and quality of life.
Studies that evaluated only the body mass index (BMI) for assessing the risk of malnutrition were excluded; books, chapters of books, case reports, editorial letters, animal studies were also excluded.
The titles and abstracts of studies identified as potentially relevant based on the inclusion and exclusion criteria were independently assessed by two researchers (MS, SM). Potentially admissible studies were finally selected by analyzing the full-text articles.
The data relating to complete search algorithm and the assessment of the risk of bias and the methodological quality of the articles included are available at the following link: https://figshare.com/s/420ada20a9e78f510d7f ; PRISMA Flow Chart are available at the following link: https://figshare.com/s/956950aa9d36cbbb2016.
Results. A total of 1,722 articles were identified: 1,596 through electronic database searches (35 Cochrane Library, 165 PubMed, 594 Embase, 353 Cinahl, 227 Scopus, 222 Web of Science) and 126 from manual searches and grey literature.
After 793 articles were deleted as duplicates, all titles were screened and 202 articles were retained and evaluated for eligibility through reading the abstract. Of these, 126 were judged not relevant and 76 full-texts were evaluated, 56 of which were further deleted as irrelevant.
After deleting duplicates and evaluating for eligibility through reading the abstract and full-texts, the screening process ultimately included 20 studies in this review (Data availability statement).
General characteristics of studies included
All the studies included in the analysis were observational. Specifically, eleven were cross-sectional studies [22, 30, 31, 32, 34, 36, 37, 38, 40, 45, 47] (55%), five prospective cohort studies [33, 35, 42, 44, 46] (25%), two retrospective cohort studies [39, 43] (10%) and two retrospective cross-sectional studies [29, 41] (10%). Only three studies were multicenter [31, 45, 46]. No interventional studies were found.
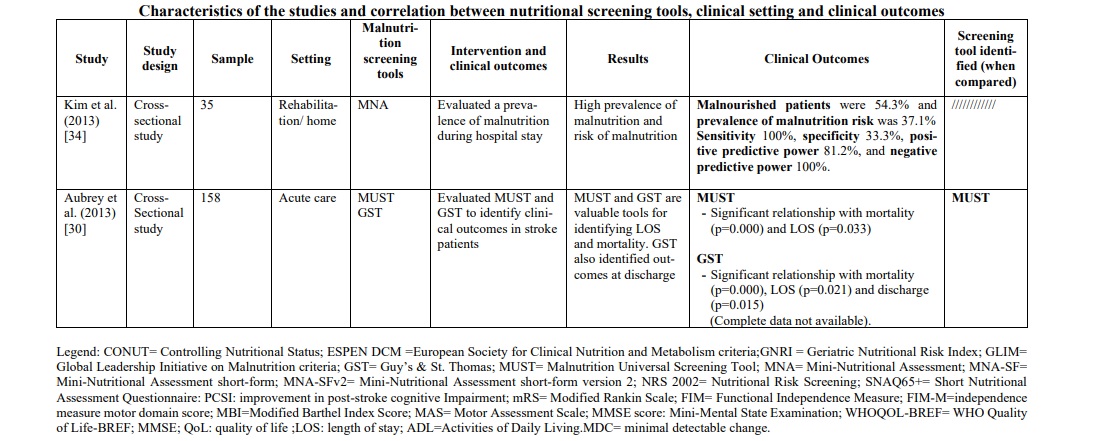
The diagnoses reported were mainly ischemic stroke and hemorrhagic stroke. The total number of stroke patients who were assessed for malnutrition was 7,029 (range 35-1906 patients per study). The correlation between the risk or the presence of malnutrition and different outcomes [prognosis and mortality [29, 30, 31, 33, 42, 47] (30%), cognitive impairment [35, 43] (10%), prevalence of malnutrition and risk predictors [22, 32, 38, 39, 45, 47] (30%), post-discharge destination [40] (5%), quality of life and subjective assessment [34, 36] (15%) and functional outcomes [41, 44, 46] (10%)] were evaluated. The main features and findings of the included studies are described in Table 1.
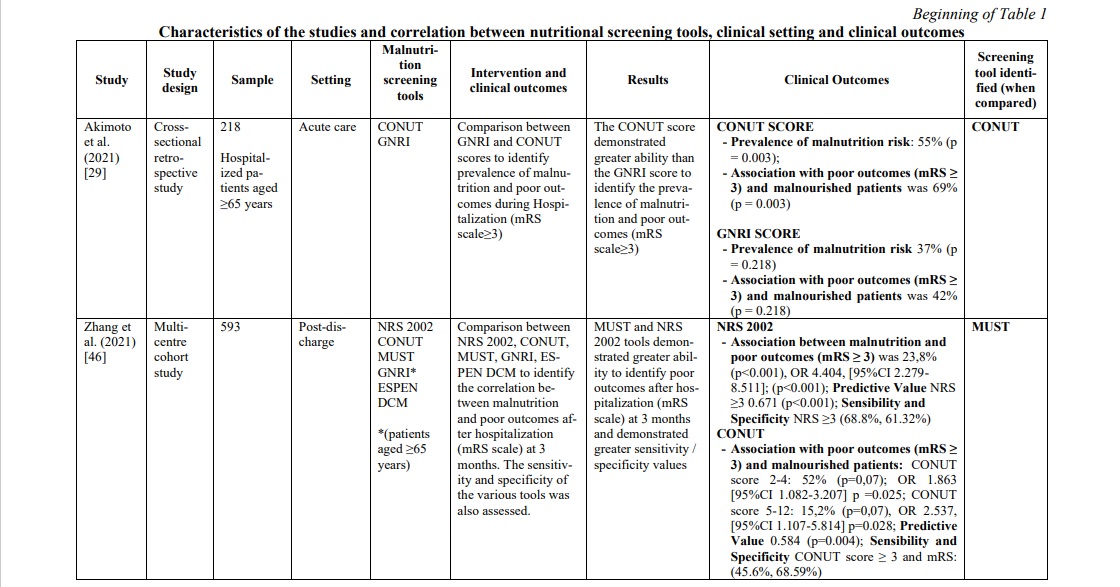
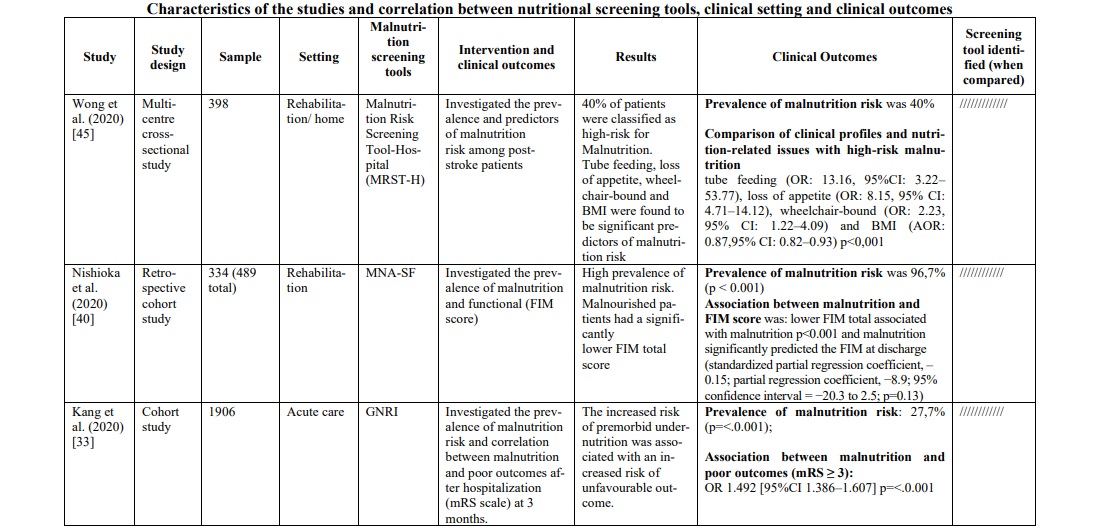
Screening tools used for nutritional evaluation
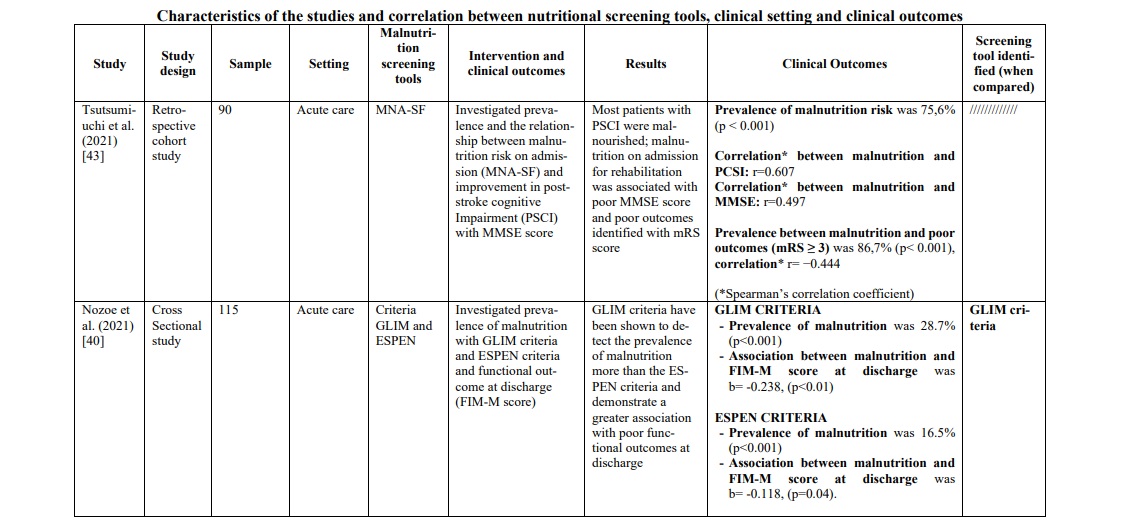
Screening/evaluation tools and anthropometric/biochemical measurements were used for nutritional examination. The risk assessment/prevalence of malnutrition was investigated by anthropometric/ biochemical measurements and different screening tools: MNA in seven studies [32, 34, 36, 37, 38, 44, 47], Geriatric Nutritional Risk Index (GNRI) in five studies [29, 33, 35, 41, 46], Controlling Nutritional Status (CONUT) in three studies [29, 31, 46], NRS 2002 in three studies [22, 32, 46], MUST in three studies [30, 42, 46], MNA-SF in three studies [36, 39, 43], The European Society of Clinical Nutrition and Metabolism-Diagnostic Criteria for Malnutrition (ESPEN DCM) in two studies [40, 46], Malnutrition Risk Screening Tool-Hospital (MRST-H) in one study [45], Global Leadership Initiative on Malnutrition (GLIM criteria) in one study [40], Guy's & St. Thomas Malnutrition Screening Tool (GST) in one study [30] and Short Nutritional Assessment Questionnaire (SNAQ65+) in one study [47]. More details on the screening tools used for the nutritional examination are shown in Table 1.
Description of the screening tools evaluated
Controlling Nutritional Status (CONUT) [29, 31, 46] is a nutritional scoring tool that is calculated using serum albumin, total cholesterol level, and total lymphocyte count.
Geriatric Nutritional Risk Index (GNRI) [29, 33, 35, 41, 46] is a nutritional scoring calculated based on the serum albumin level and the ratio of present body weight to ideal body weight. The GNRI was proposed for assessing the nutritional status of elderly patients with various illnesses.
Global Leadership Initiative on Malnutrition (GLIM criteria) [40] includes three phenotypic criteria (weight loss, low body mass index, and decreased muscle mass) and two etiological criteria (decreased food intake or absorption and increased disease burden or inflammation). If a patient meets at least one phenotypic criterion and one etiological criterion, malnutrition is diagnosed.
Guy's & St. Thomas Malnutrition Screening Tool (GST) [30] is a rapid screening tool that evaluates BMI, recent weight loss and dietary intake. The tool categorises patients in low, medium and high risk of malnutrition.
Malnutrition Risk Screening Tool-Hospital (MRST-H) [45] is a validated tool for nutritional screening that evaluates financial dependence, food dependence, unintentional weight loss, and measures of mid-upper arm circumference and calf circumference of the non-paralytic limb. The score classifies patients at high risk of malnutrition (≥ 2) or low risk of malnutrition (< 2).
Malnutrition Universal Screening Tool (MUST) is a five-step screening tool to identify adults who are malnourished, at risk of malnutrition (undernutrition), or obese. It also includes management guidelines, which can be used to develop a care plan. It is suited for hospitals, community and other care settings and can be used by all care workers. The total score identifies whether the patient has a low (0 point), medium (1 point) or high (≥ 2 points) risk of malnutrition.
Mini Nutritional Assessment (MNA) [32, 34, 36, 37, 38, 44, 47] is a validated nutrition screening and assessment tool that can identify geriatric patients (aged 65 and above) who are malnourished or at risk of malnutrition. The 18-item MNA includes anthropometric measurement, global assessment, dietary questionnaire, and subjective assessment. The MNA identify patients well-nourished (score 24-30), at risk for malnutrition (score 17–23.5) and malnourished (< 17).
Mini Nutritional Assessment short-form (MNA-sf) [36, 39, 43] consists of 6 questions highly correlated with the original 18-item long form, which should be used if further investigations into the client’s condition are warranted after administration of the MNA-sf. Each item of the MNA-sf is scored along a scale 0-3 with total scores ranging 0-14. The assessment takes less than 10 minutes to complete with lower scores suggestive of poorer nutritional intake.
Nutritional Risk Screening 2002 (NRS 2002) [22, 32, 46] is a validated tool for nutritional screening of patients aged from 18 to 90 years who have or are at risk of malnutrition. The tool includes standard screening parameters, such as body mass index (BMI), patient’s age, weight loss, dietary intake, and severity of underlying disease. The NRS-2002 score ranges from 0 to 7, and a total score ≥ 3 indicates a patient “at nutritional risk”
Short Nutritional Assessment Questionnaire (SNAQ65+) [47] uses a set of quick and easy-to-apply criteria (recent weight loss, ability to climb stairs, appetite, mid-upper arm circumference). This tool is used not only in hospitals, but also in home care.
The European Society of Clinical Nutrition and Metabolism-Diagnostic Criteria for Malnutrition (ESPEN DCM) [40, 46] has two alternatives for diagnosing malnutrition after initial nutritional screening with a validated screening tool. One is to diagnose malnutrition if the BMI is <18.5 kg/m2, and the other is a combination of unintentional weight loss (>10% of habitual weight indefinitely or >5% over 3 months), reduced BMI (<20 kg/m2 for patients aged <70 years, or < 22 kg/m2 for patients aged >70 years), or low FFM index (<17 kg/m2 in men and <15 kg/m2 in women).
Clinical context of application of nutritional screening tools
The nutritional risk assessment scales or scores used in the included studies were classified according to the context of application and divided into acute, post-acute (outpatient) and rehabilitation/home, respectively described in 17 (56.7%), 8 (26.7%) and 5 (16.6%) studies.
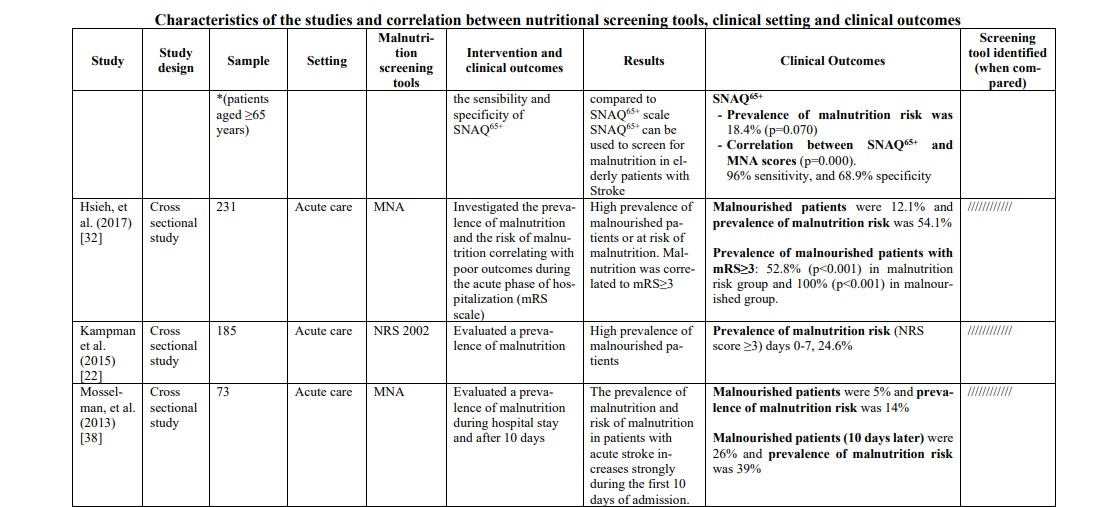
In the hospital context, in which patients are mainly affected by stroke in the acute phase, the most frequently used screening tool was the GNRI, used in three studies [29, 33, 41] accounting for 33.1% of the total sample of patients analyzed, followed by the MUST (two studies [30, 42], 17.9%), the CONUT (two studies [30, 31], 11.2%), NRS 2002 (two studies [18, 31], 10.7%) and MNA (three studies [36, 42, 48], 4.8%).
In the post-acute phase, the most frequently used screening tools were the CONUT, used in a single study [46] accounting for 8.4% of the total sample of patients analyzed, followed by the GNRI (two studies [35, 46], 6.4%), the NRS 2002 used in a single study [46] (3.7%), the MUST used in a single study [46] (3.1%), the SNAQ65+ and the MNA used in a single study [47].
Finally, in the context of rehabilitation/home, the nutritional screening tools identified were the MNA-SF used in two studies [37, 39] accounting for 8% of the total sample analyzed, the MRST-H used in one study [45] (5.66%), and the MNA, used in three studies [36, 37, 44] (2.6%) (Table 1).
Screening tools, clinical outcomes and clinical context
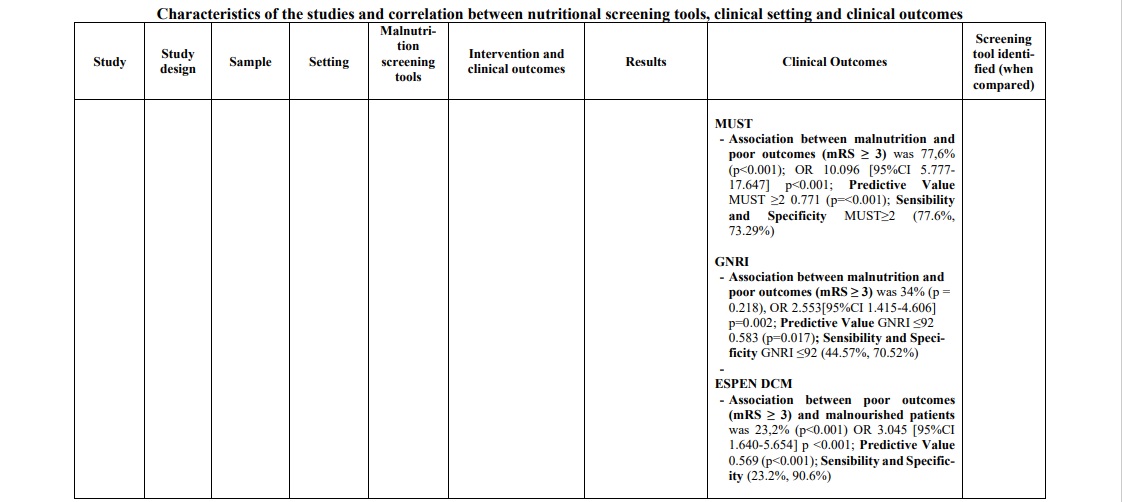
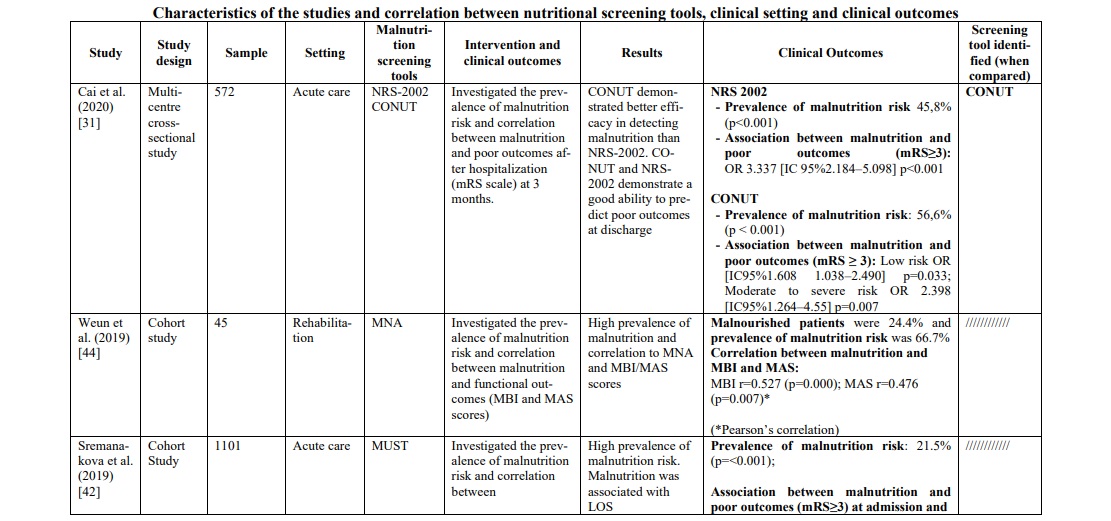
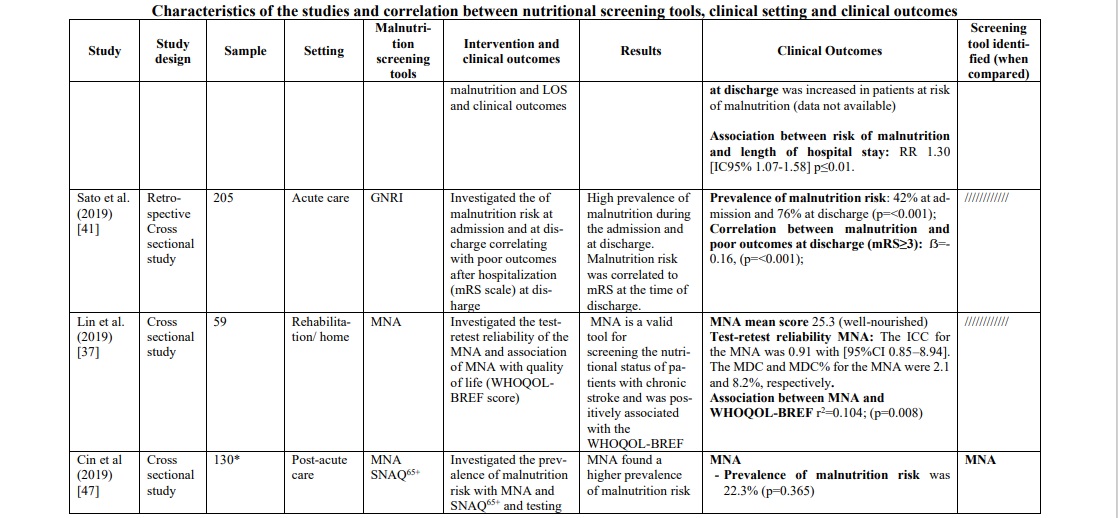
In the acute phase of stroke, the following three tools were mostly applied in the hospital context: GNRI, MUST and CONUT. GNRI proved to be valid to detect malnutrition [35, 41] in this population (range 17.2% - 76%), as well as MUST [42, 46] (range 21.5% - 77.6%) and CONUT [29, 31] (range 55% - 56.6%). To support these data, two studies [29, 46] compared the three tools showing a superiority of CONUT over GNRI in a first study [29] (prevalence of malnutrition CONUT score=55% p=0.003; prevalence of malnutrition GNRI score= 37% p=0.218), while in the second one [46] MUST demonstrated significantly superior data compared to the other two tools (prevalence of malnutrition MUST=77,6% p<0.001; prevalence of malnutrition CONUT score=67,2% p=0.007; prevalence of malnutrition GNRI score= 34% p=0.218). Furthermore, in the latter [46] and in other studies [29, 31, 33, 41, 42], the correlation between malnutrition and poor clinical outcomes was measured with the mRS score (Modified Rankin Scale), and also in this correlation CONUT score was better than GNRI score. However, when the analysis included also the MUST [46] scale, the correlation was clearly in favor of the latter [(Pearson r=0.776, p<0.001); (OR 10,096, 95% CI 5,777-17,647, p<0.001); (Predictive Value MUST ≥2 0.771, p<0.001); (Sensibility and Specificity MUST ≥2 77.6%, 73.29%)]. To support this, MUST scale showed an association between risk of malnutrition and length of hospital stay (RR 1.30 (CI95% 1.07-1.58) p≤ 0.01) [42] and statistically significant relationship with mortality (p<0.0001) and Lenght of stay (LOS) (p=0.033) [30].
Finally, only one study [35] analyzed the correlation between malnutrition (assessed through GNRI) and hospital discharge, evaluating the association between malnutrition and cognitive impairment measured with PCSI (improvement in post-stroke cognitive impairment) and MMSE (Mini-Mental State Examination). Low GNRI is associated with an increased risk of PSCI (OR, 2.04; 95% CI, 1.00–4.14; p=0.049), and a correlation between low GNRI and MMSE is present (z-scores ẞ=0.04, p=0.03) (Table 1).
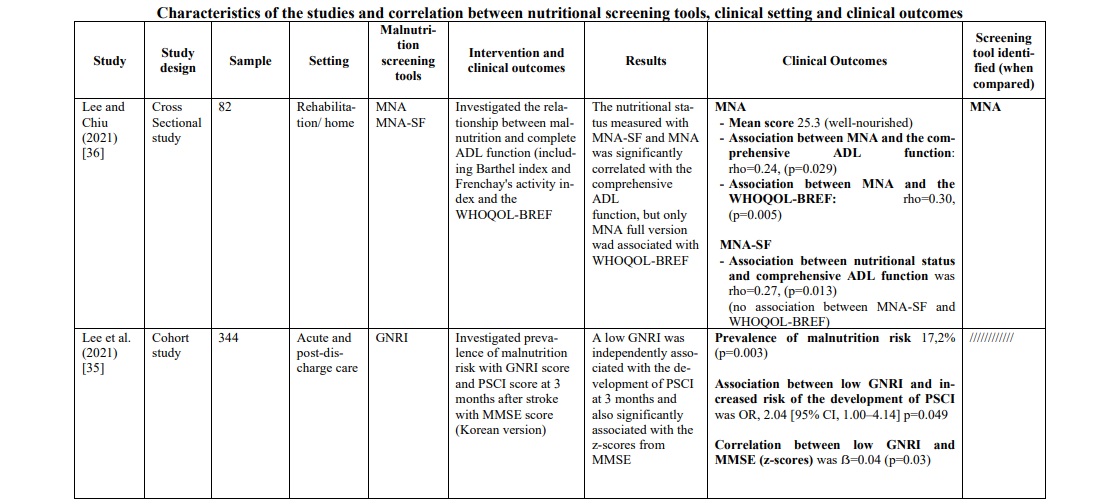
In the rehabilitation/home setting, most studies [34, 37, 40, 44-47] used the MNA screening tools (full or short version). The prevalence of malnutrition was between 22,3% and 96,7%. It was not possible to compare these data with those of other screening tools, except in one study [47], in which MNA was compared with the SNAQ65+ tool, demonstrating a greater ability of MNA to identify patients at risk. To support this, the study by Kim et al.34 found that MNA, applied in patients suffering from chronic stroke, showed sensitivity 100%, specificity 33.3%, positive predictive power 81.2%, and negative predictive power 100%. Furthermore, when MNA was compared with the MNA-SF version, it proved to be superior, in particular when correlated with quality of life (QoL) measured with the WHOQOL-BREF [36] instrument (WHO Quality of Life-BREF) (rho=0.30, p=0.005). These data were confirmed by a second study [37] (r2=0.104; p=0.008), which also evaluated the reliability of the instrument through test-retest (ICC 0.91, [CI95% 0.85-8.94], minimal detectable change was 2.1 and 8.2%).
MNA also demonstrated a strong association towards functional outcomes [44] such as MBI score (Modified Barthel Index Score) (r=0.527, p<0.001) and MAS score (Motor Assessment Scale) (r=0.476, p=0.007), ADL (Activities of Daily Living) [36] (rho=0.24, p=0.029) and functional outcomes at discharge [40] (Table 1).
Discussion. This systematic review aimed to identify which malnutrition risk assessment tools are applicable to post-stroke patients and in which stage of the disease (acute or chronic) and setting of care (hospital, rehabilitation or home) are applicable.
In the hospital setting (acute/post-acute phase), the most widely used screening tools were the Geriatric Nutritional Risk Index (GNRI), the Malnutrition Universal Screening Tool (MUST), and the Controlling Nutritional Status (CONUT). All these tools proved to be applicable to the population under investigation and able to adequately identify the nutritional risk. Considering the clinical outcomes of interest, the MUST scale demonstrated a greater capacity than the other two scores in detecting malnutrition and relevant correlated clinical outcomes such as mortality, length of stay, outcomes at discharge, functional capacity and quality of life. As known in the literature, malnutrition is directly correlated with poor clinical outcomes [3] and our research also investigated whether the different nutritional risk assessment scales correlated with poor clinical outcomes during hospitalization. Also in this evaluation, the MUST scale proved to be superior when compared with the different tools investigated [30, 46], demonstrating a positive correlation with clinical outcomes such as: length of hospital stay (RR 1.30 (CI95% 1.07-1.58) p≤ 0.01), mortality (p=0.000) and LOS (p=0.033) [30]. Moreover, when correlated with functional disability measured with the mRS scale, MUST showed a significant association equal to 77.6% p<0.001, demonstrating sensibility and specificity respectively 77.6% and 73.29% [42]. In support of this tool there is certainly also the rapidity and easiness of administration that make this nutritional risk assessment scale applicable in hospital clinical setting [3].
Differently, in the rehabilitation/home setting our research has identified only one instrument of interest, the Mini Nutritional Assessment (MNA and MNA-SF version). The use of this tool in this population is dictated by the fact that it was created for application in an elderly population and gracefully in a rehabilitation/home context [34, 36, 43]. The study by Kim et al. [34] showed that MNA, applied in a sample of chronic stroke patients, showed a sensitivity of 100%, a specificity of 33.3%, a positive predictive value of 81.2% and a negative predictive value of 100%; although they demonstrated adequate diagnostic accuracy, these data were obtained from a relatively small sample size (n=35). Furthermore, MNA has been shown to be superior to MNA-SF in detecting the risk of malnutrition and in particular when correlated with clinical outcomes of fundamental importance in a chronic care setting such as quality of life (QoL) assessment measured with the WHOQOL-BREF [36, 37] instrument and in others functional outcomes such as MBI score, MAS[44] score and ADL[36]; This evidence is supported by a study [37], that evaluated the very reliability of the instrument through a test-retest study on a sample of patients with chronic stroke, defining an ICC value of this instrument equal to 0.91.
Conclusions. Since stroke is one of the main causes of death, especially when associated with malnutrition, an assessment of nutritional status in all stages of the disease is important. However, not all tools, especially if they were not developed for this specific population, may be suitable for the early identification of malnutrition.
This systematic review of the literature is the first to evaluate the application of nutritional assessment scales in the post-stroke patient, specifically investigating which tool was most suitable for the different phases of the disease. In our opinion, this factor is of fundamental importance because the various screening tools have been created and validated on specific populations. Our results have not only confirmed what is indicated by the ESPEN, indicating the MUST as the tool to be applied to the patient with stroke in the acute phase of the disease, because this scale has shown not only greater capacity in identifying malnutrition when compared with all the tools investigated, but also a correlation with poor clinical outcomes, such as mortality, LOS and functional disability.
In the chronic phase of stroke, MNA full version has shown not only to be the most widely applied scale in almost all studies in this setting, but from our research we have obtained sufficient data to indicate that this tool can be the reference tool in the chronic stroke patient. Our considerations are supported by several studies, mainly observational and cohort studies that have evaluated adequate patient samples and demonstrating that MNA when compared with other tools has shown greater ability to identify nutritional risk, a characteristic supported by a high sensitivity and predictiveness of this tool which also showed adequate results when applied in a test-retest study. Furthermore, MNA demonstrated a strong correlation with clinical outcomes of relevance for the chronic population investigated, as QoL, functional outcomes and ADL.
This research aims to be a valid resource to researchers and clinicians for the assessment of malnutrition risk in the different phases of post-stroke.
Financial support
No financial support has been provided for this work





















Reference lists