Regulation of natural killer cells cytotoxicity by trophoblast cells: proteins and receptors
Abstract
Background: Interaction between trophoblast and natural killer cells is determined by microenvironment. Cytotoxicity of NK against targets, including trophoblasts, is associated with production of perforin, granzymes. Trophoblasts are not destroyed by NK but trophoblast-cell resistance is understudied. The aim of the study:To evaluate the interaction between NK and trophoblasts in the presence of cytokines typical of the uteroplacental contact microenvironment during pregnancy. We evaluated changes in the expression of proteins responsible for the implementation of cytotoxic function in NK and trophoblast cells under short-term and long-term co-culture. Materials and methods: In this study we used cell cultures – NK-92 and JEG-3 as a model of interaction between fetus and mother’s immune system. To analyze changes in phenotype, cytokines secretion and amount of cytotoxicity proteins we used flow cytometry. Results: We observed that intensity of GrA expression by NK-92 was lower than GrB. There was no transfer of GrA to trophoblast within 24 hours of co-culturing. The appearance of GrA in trophoblast with a simultaneous decrease in expression by NK-92 was noted after 96 hours. NK-92 and JEG-3 cells constitutively expressed serpin B9. Cytokines did not affect the content of GrA, GrB, perforin, and serpin B9 in the NK-92 monoculture. IL-15, IL-18, IL-10 caused a decrease in serpin B9 expression by trophoblasts, but co-culturing with NK-92 diminished this effect. When co-cultured with trophoblasts for 96 hours, intensity of expression of GrA, GrB, perforin and serpin B9 by NK-92 decreased. TNFα and IL-15 decreased the inhibitory effect of trophoblasts on GrA expression by NK. IL-15 and TGFβ caused an increase in the number of JEG-3 containing GrA. Conclusion: The interaction between NK and trophoblasts is a dynamic process accompanied by the transfer of cytotoxic proteins and changes in the expression of serpin B9. The used cytokines affected the transfer of proapoptotic proteins in co-culture
Keywords: granzyme, perforin, serpin B9, trophoblast, NK-cells, HLA-G, IL-10, IL-15, IL-18, TNFα, IFNγ, TGFβ1
Introduction. The main function of natural killer cells (NK cells) is to realize cytotoxicity against malignant or virus-infected cells [1]. The role of NK cells in regulating the interaction between immune cells and the microenvironments, including during pregnancy, has been actively studied [2, 3, 4]. Decidual NK (dNK) cells differ in their phenotype and function from peripheral blood NK cells [2]. dNK cells interact with trophoblast cells, which invade the decidua. It is assumed that trophoblast cells and dNK cells influence each other's characteristics due to contact and distant interactions [2, 5]. Trophoblast and NK cells secrete a wide range of cytokines in the uterus to regulate the functions of each other as well as the microenvironment. Both trophoblast and NK cells have been proven to secrete TGFβ [6, 7]. It has been observed that TGFβ suppresses the ability of NK cells to proliferate, inhibiting their cytotoxicity [8]. TGFβ also stimulates trophoblast-cell invasion [9]. The functions of the microenvironment in the uteroplacental contact zone can be controlled by TGFβ as well as TNFα, IFNγ, IL-10, IL-18, and IL-15; these are secreted by trophoblast cells, NK cells, and other microenvironment cells [10-13]. IL-15 and IL-10 are produced by trophoblast, stromal, and dendritic cells; IL-18 is produced both by macrophages and endometrial cells [14, 15].
NK cells realize cytotoxicity against target cells, including trophoblast cells, in several ways. One is the secretion of granzymes, granulysin, and perforin [16]. dNK cells have reduced cytotoxicity [17]. This may be caused by several factors, including a decrease in the activity of cytotoxic protein synthesis under the influence of microenvironmental factors (trophoblast cells and cytokines) or the transfer of a part of cytotoxic proteins to nearby cells [18]. It has been demonstrated that the cytotoxic function of NK cells changes under the action of cytokines with an increase in exposure time. Cо-culturing in the presence of TNFα for 24 hours increased the cytotoxicity of NK cells toward trophoblasts; a prolongation of the co-culture time diminished this effect. Different results were obtained for TGFβ[19]. Despite their reduced cytotoxicity, dNK cells should retain a baseline level; a decrease may cause obstetric pathologies.
It is believed that one method to restore the dNK cell pool is the migration of NK cells from peripheral blood. It has been observed that in patients with recurrent implantation failures, peripheral blood NK cells have reduced cytotoxicity as well as a low expression of activating receptors and granzyme B (GrB) compared with fertile women [20].
Serpins are inhibitors of serine proteinases. They regulate the activity of granzymes and protect target cells from their action. The interaction between serpin B9 and GrB is the most studied [7]. Another protective mechanism of trophoblast cells is their expression of a nonclassical MHC class I molecule, HLA-G [21]. HLA-G binds to the receptor on the NK cell surface, causing a decrease in the synthesis of IFNγ by NK cells [22] as well as their cytotoxic activity [23]. The reduced cytotoxicity of uterine dNK cells may be caused by their differentiation from regulatory phenotypes under the effect of trophoblast cells. Populations of NK cells with reduced levels of CD56 tend to have a greater cytotoxic activity[23], whereas NK cells of the decidua have increased CD56 levels and a low cytotoxic activity [24]. An increase in CD56 expression was considered to be a marker of NK cell differentiation in a decidual population [25]. Trophoblast cells may be regulators of this transformation; the intensity of CD56 expression levels by NK cells increased when they were co-cultured [26].
During pregnancy, a unique balanced system develops in the uterus between NK, trophoblast, and other cells of the microenvironment. This is necessary for the maintenance and development of physiological pregnancy [27]. Despite the importance of these processes that occur in the uterus during pregnancy, the molecular mechanisms of the interaction between NK cells and trophoblast cells have not been fully studied. This may be due to both the complexity of dNK cell isolation and the difficulties associated with the modeling of cell interactions.
The aim of the study. To evaluate the interaction between NK and trophoblasts in the presence of cytokines typical of the uteroplacental contact microenvironment during pregnancy. We considered changes in the expression of the proteins to be responsible for the realization of the cytotoxic function by NK cells. We also evaluated the content of these proteins in trophoblast cells after their short-term and long-term co-culture in the presence of cytokines. Information regarding the content of cytokines and proteins in the natural killer or trophoblast cells used in this work and their functions are described in Table 1.
Materials and Methods. We performed this study using JEG-3 and NK-92 cell lines (ATCC, USA), which reflect the main characteristics of extravillous trophoblasts and natural killer cells, respectively [97, 98, 99]. The cells were cultured according to the manufacturer's recommendations in a humid environment at 37 °C with 5% CO2. The viability of the cells was controlled by trypan blue exclusion; this was 95±3.4%.
The following inductors were used: TNFα (50 U/mL), IFNγ (1000 U/mL), TGFβ1 (5 ng/mL), IL-15 (10 ng/mL), IL-18 (10 ng/mL), and IL-10 (10 ng/mL) (RnD, USA). These concentrations were chosen according to the concentrations of human biological fluids, including in the area of uteroplacental contact [100, 101, 102]. Cultivation of NK-92 cells in a monoculture or co-culture with JEG-3 cells. The cells were cultivated as previously described in [103]. Briefly, 2×104 cells of the JEG-3 line were cultured in the wells of a 96-well plate (Sarstedt, Germany) in 100 µL of a complete growth medium (DMEM) (Biolot, Russia). After 24 hours, NK-92 cells were added to parts of the wells, with a formed monolayer of trophoblast cells in 100 µL of a medium of 20,000 cells (the co-culture). NK-92 cells were also placed in empty wells to receive a monoculture. IL-2 (500 U/mL) was added to all wells (Biotech, Russia). Cytokines were added to a few of the wells, then the cells were cultured for 24 hours in the presence of TGFβ1 or 96 hours in the presence of TNFα, IFNγ, TGFβ1, IL-15, IL-18, or IL-10. Subsequently, analyses of the cell expressions of the surface receptors and intracellular proteins were conducted. Four experiments were conducted, with two technical repetitions for each experiment.
Analysis of the surface and intracellular protein levels in NK-92 and JEG-3 cells under mono- and co-culture conditions. After the co-cultivation of JEG-3 and NK-92 cells, fixation and permeabilization were performed for 24 and 96 hours using a Cytofix/Cytoperm kit (BD, USA). GrA, GrB, perforin (BD, USA), and serpin B9 (NOVUS, USA) monoclonal antibodies were used for intracellular staining according to the manufacturers’ instructions. To separate the NK cells and trophoblast cells, we used CD45, CD56, cytokeratin 7 (BD, USA), and HLA-G antibodies (Biolegend, USA). The level of expression of HLA-G by the JEG-3 cells was also analyzed. The control of nonspecific binding was identified using isotypic antibodies. We studied two parameters: the relative number of cells that expressed/contained proteins and the mean fluorescence intensity (MFI). These parameters were assessed using a FacsCantoII flow cytometer (BD, USA). We did not observe any data to confirm that JEG-3 cells could spontaneously express cytotoxic-related proteins. We used “expression” to describe changes in the content of GrA, GrB, and perforin in JEG-3 cells. The gating strategy is presented in Supplementary Materials, Figure 1.
Statistical analysis. Statistical analysis of the data obtained was performed using a non-parametric analogue of the Mann-Whitney test with Bonferroni correction. For multiple comparisons, we used the Kruskal-Wallis one-way analysis of variance. All tests were performed using GraphPad Prism, version 8.0.0 (GraphPad Software, San Diego, California, USA).
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Federal State Budgetary Scientific Institution “D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology” (Protocol No.118, 9 June 2022).
Results and discussion
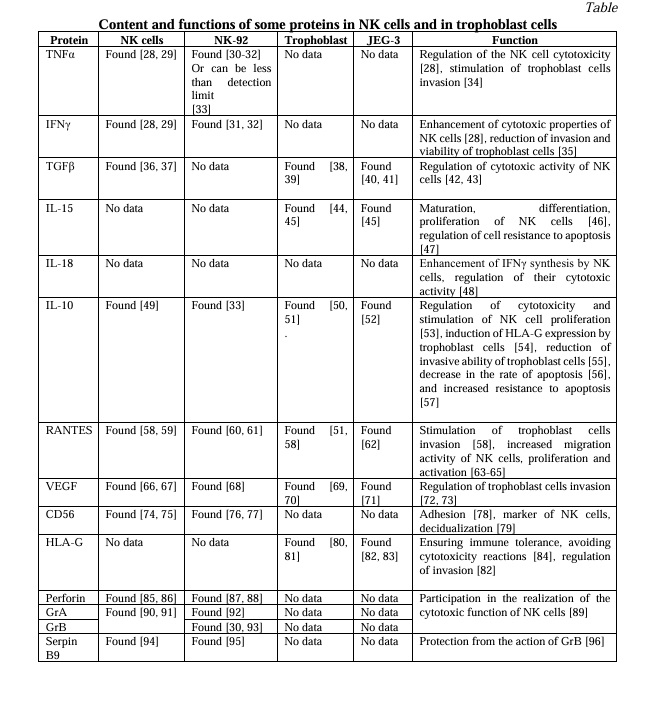
Evaluation of HLA-G expression by JEG-3 cells after cultivation for 24 and 96 hours. Under mono- and co-culture conditions for 24 hours, the JEG-3 cells did not change the expression of HLA-G (both the relative number of cells and the MFI) in the presence or absence of TGFβ1 (Fig. 1a, 1b).
In monoculture conditions (96 hours), the relative number of JEG-3 trophoblast cells expressing HLA-G and the intensity of the HLA-G expression were higher in the presence of TGFβ1 compared with the trophoblast cells cultivated without it (Fig. 1c, 1d). The other cytokines in the monoculture (96 hours) did not affect the expression of HLA-G by the trophoblast cells. In the presence of TNFα (96 hours), the number of trophoblast cells expressing HLA-G was higher in co-culture conditions with NK-92 cells compared with trophoblast cells in a co-culture without cytokines as well as in a monoculture with cytokines (Fig. 1c). In the presence of TNFα (96 hours), the intensity of the expression of HLA-G by JEG-3 cells in the co-culture was higher than without cytokines, but did not differ from the monoculture of the same cells with cytokines (Fig. 1d).
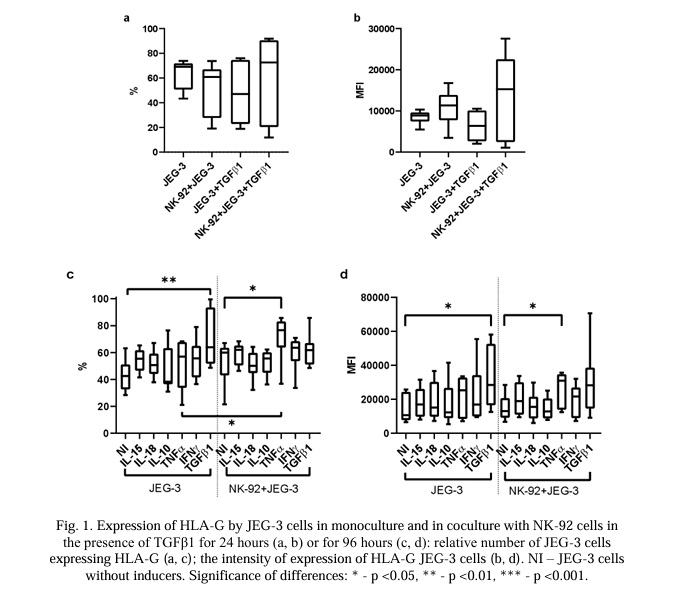
Expression level of cytotoxicity-related proteins in NK cells and trophoblast cells after co-cultivation with or without TGFβ1 for 24 hours. The NK-92 cells contained the cytotoxic proteins GrA, GrB, and perforin (Fig. 2). Neither the number of NK-92 cells containing GrA and perforin nor the intensity of expression of these proteins changed under different culture conditions for 24 hours (Fig. 2). Under the same conditions, the number of NK-92 cells containing GrB also did not change (Fig. 2b). However, the intensity of the GrB expression was reduced after the incubation of NK cells with trophoblast cells compared with the monoculture. This difference was established both in cultivation in an environment with TGFβ1 and without it (Fig. 2e)
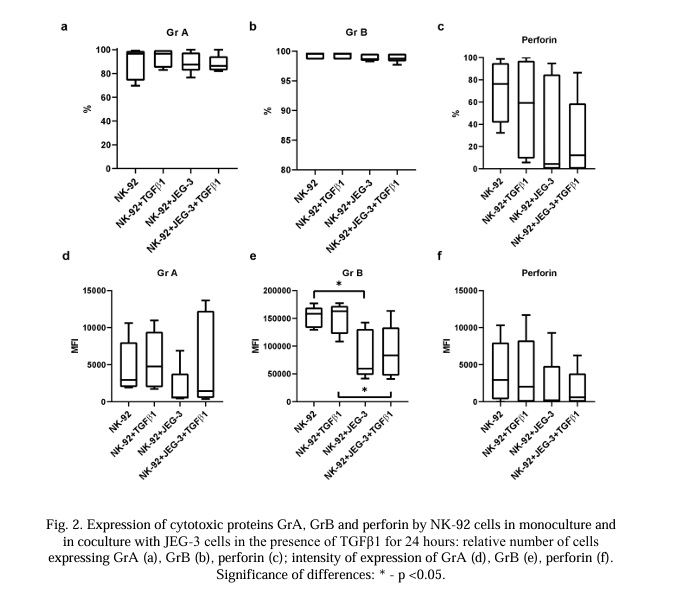
JEG-3 cells did not express GrA, GrB, or perforin (Fig. 3 and 7). In the conditions of a co-culture with NK-92 cells, the number of JEG-3 cells expressing GrB increased within 24 hours (Fig. 3b). These results were established both in the presence of TGFβ1 and without it in co-culture conditions. Under the conditions of a co-culture with trophoblast cells, the intensity of the expression of GrB and perforin increased both in the medium with TGFβ1 and without it (Fig. 3e, 3f).
The number of NK cells expressing serpin B9 (Fig. 4a) and the intensity of its expression by NK cells (Fig. 4b) were reduced in co-culture conditions compared with a monoculture of NK-92 cells. After 24 hours of incubation with NK-92 cells, neither the number of trophoblast cells expressing this protein (Fig. 4c) nor the intensity of the serpin B9 expression (Fig. 4d) changed.
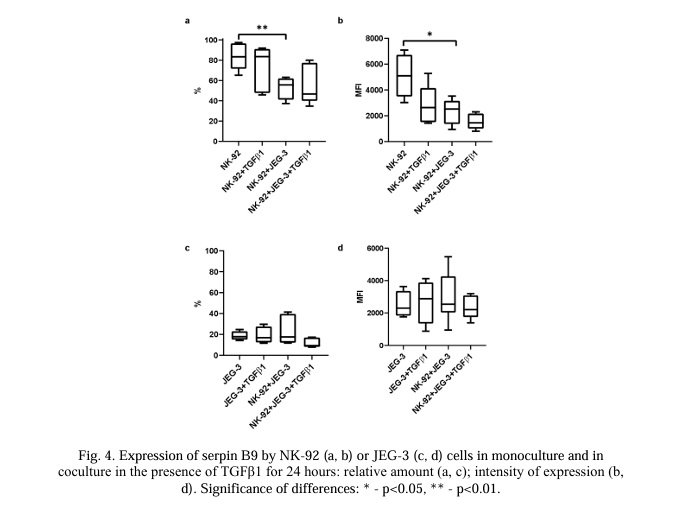
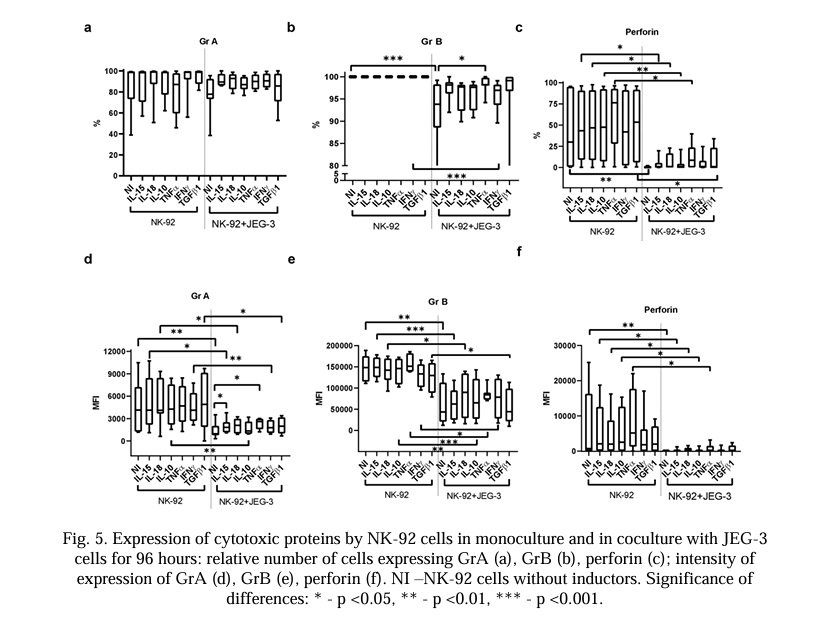
Content of cytotoxicity-related proteins in NK and trophoblast cells in conditions of their mono- and co-culture for 96 hours in the presence of cytokines. After 96 hours in the co-culture, the relative number of NK-92 cells containing GrA did not change in comparison with the monoculture.
Cytokines did not affect the number of NK-92 cells expressing GrA in the monoculture or in the co-culture with trophoblast cells (Fig. 5a). Cytokines did not affect the intensity of the GrB expression by the NK cells under monoculture conditions. The intensity of the GrA expression in NK-92 cells was lower in the presence of JEG-3 trophoblast cells compared with nonactivated cells (Fig. 5d). In the presence of the cytokines IL-15, IL-18, IL-10, IFNγ, and TGFβ1, the intensity of the GrA expression by the NK-92 cells was lower in the co-culture relative to the NK cells in the presence of corresponding cytokines in the monoculture (Fig. 6d). IL-15 and TNFα caused an increase in the intensity of the GrA expression in the NK-92 cells in the co-culture compared with the NK cells in the co-culture without cytokines (Fig. 5d).
Cytokines did not affect the number of NK-92 cells expressing GrB or the intensity of protein in the monoculture. The relative number of NK cells expressing GrB was lower in the co-culture without cytokines and in the presence of IFNγ; other cytokines did not affect this parameter. TNFα canceled the effect of the trophoblast cells on the expression of GrB by the NK cells in the co-culture (Fig. 5b). The intensity of the GrB expression in the NK-92 cells was lower in the co-culture without cytokines. Cytokines did not affect the intensity of the GrB expression in the NK cells in the co-culture with trophoblasts (Fig. 5e).
The relative number of NK-92 cells expressing perforin was reduced in co-culture conditions compared with the NK cells in the monoculture (Fig. 5c). This effect did not change when cytokines were added to the system (Fig. 5c). The intensity of the perforin expression in the NK-92 cells was reduced in the co-culture conditions compared with its expression in the NK-92 cells cultured in the monoculture. This effect did not change in the presence of cytokines (Fig. 5f).
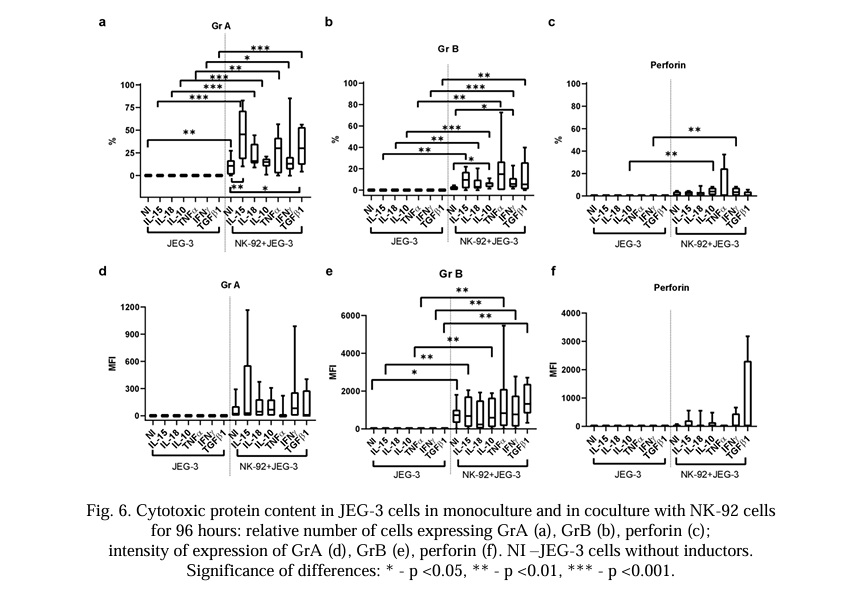
The relative number of JEG-3 cells containing GrA increased in the presence of the NK-92 cells. Of all the cytokines used in the co-culture, only IL-15 and TGFβ1 caused an increase in the relative number of JEG-3 cells containing GrA compared with intact cells (Fig. 6a).
The relative number of JEG-3 cells containing GrB did not change under the conditions of the co-culture. There was an increase in the intensity of the GrB expression by the trophoblast cells in the co-culture conditions (Fig. 6b, 6e). Under co-culture conditions, only IL-10 and IFNγ increased the relative number of JEG-3 cells expressing GrB compared with intact cells in the co-culture.
The relative number of JEG-3 cells containing perforin and its MFI did not change under co-cultivation conditions with NK cells. Cytokines did not affect the MFI of perforin, either in the monoculture or the co-culture. The relative number of JEG-3 cells containing perforin was higher in the co-culture with NK cells with the addition of IL-10 or IFNγ compared with the trophoblasts in the monoculture with these cytokines, but did not differ from the trophoblast cells in the intact co-culture (Fig. 6c, 6f).
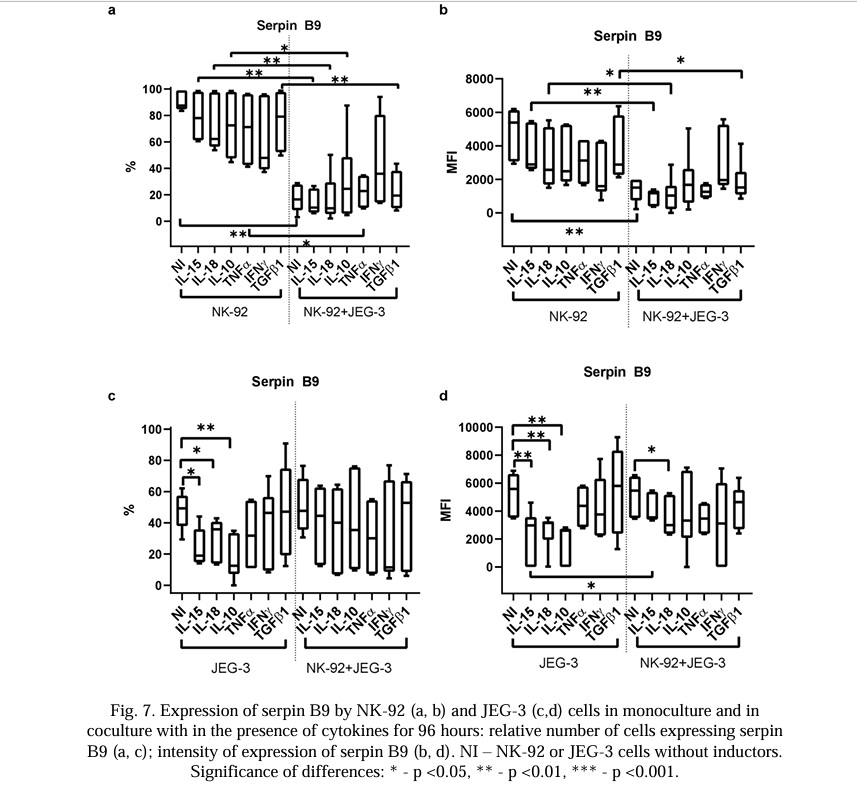
The NK-92 and JEG-3 cells expressed serpin B9 at a base level. Cytokines did not affect the number of NK cells expressing serpin B9 or the intensity of the serpin B9 expression in the monoculture. The relative number of NK-92 cells containing serpin B9 and its MFI were reduced in the co-culture compared with the intact NK-92 cells in the monoculture. The cytokines did not change this effect (Fig. 7a, 7b). In the monoculture, the relative number of JEG-3 cells containing serpin B9 and the MFI were reduced in the presence of IL-15, IL-18, or IL-10 compared with the intact cells (Fig. 7c).
In the co-culture, the relative number of trophoblast cells and the intensity of the serpin B9 expression did not change in comparison with the monoculture. In the co-culture, the NK-92 cells canceled the reducing effect of IL-15 and IL-10 on the intensity of the serpin B9 expression by the trophoblast cells (Fig. 7c, 7d).
NK cells can realize cytotoxicity against trophoblast cells [104]. They also express death receptors such as TRAIL-R and CD95 [101, 105]. Their ligands (TRAIL and CD95L) are expressed by trophoblast cells [106, 107, 108], indicating the possibility of an induction of apoptosis in natural killer cells by trophoblast cells. Despite this, there is no total destruction of these cells in the area of uteroplacental contact. This indicates the existence of short-term and long-term programs of interactions between these cells, depending on the situation. These may include [i] the existence of checks and balances in the mode of tolerance in the mother-fetus system; [ii] a restriction of trophoblast hyper-invasion [110, 111]. We posited that the co-cultivation of NK and trophoblast cells for 24 hours may not be sufficient to demonstrate their mutual influence on the level of cytotoxic proteins and surface receptors[42]. In previous research, we have observed that long-term interaction between NK cells and trophoblast cells leads to a change in NK cell phenotype and an increase in CD56 expression [42]. The cytotoxic function of NK cells against trophoblast cells also differed after the preincubation of cells with cytokines for 24 and 96 hours[42]. Incubation for 24 hours was not enough for the mutual regulation of cells. We limited the evaluation of the level of expression of certain receptors and proteins associated with cytotoxicity. We focused on the assessment of the TGFβ effect under 24-hour co-culture conditions because TGFβ is one of the key cytokines in the uteroplacental contact zone [112] and is capable of altering the functional activity of both trophoblast cells[38, 113] and NK cells [8, 43]. We conducted an extended assessment of the effect of cytokines on the expression of the receptors and proteins associated with cytotoxicity using the cells in the monoculture and co-culture for 96 hours. According to our data, it was possible to detect the long-term effect of cells on each other at this point [42, 114-117].
In this study, we established the content of GrA, GrB, and perforin in NK-92 cells. This concurred with the data on the content of these proteins in NK cells (Table 1). We did not observe GrA, GrB, or perforin in the trophoblast cells of the JEG-3 cell line without preculturing with NK-92 cells.
The production of GrA, GrB, and perforin by NK cells is one method to realize cytotoxicity against target cells. A decrease in the content of GrA and GrB in NK cells leads to a decline in the cytotoxicity of NK cells and an increase in tumor growth [89]. Perforin is also involved in the realization of the cytotoxic properties of NK cells; this protein is necessary for the formation of pores in the membranes of the target cells that are required for Gr transport 10.1080/20013078.2019.1588538. Approximately 40% of patients suffering from malignant tumors of the blood and circulatory system have a perforin deficiency [118].
Decidual NK cells are in close contact with cells of fetal origin (including trophoblast cells). They also contain cytotoxic proteins. Despite this, dNK cells have reduced cytotoxicity compared with peripheral NK cells [17]. We noted that after co-cultivation with NK cells, perforin and GrB were observed inside JEG-3 cells within 24 hours and the amount of GrB, but not perforin, decreased in the NK-92 cells. The transfer of these proteins from NK cells to trophoblast cells was not accompanied by a decrease in cell viability. This result agreed with data previously obtained using a Western blot analysis [119].
We discovered that the intensity of the GrA expression by the NK-92 cells was ten times lower than that of GrB. There was no transfer of GrA from the NK-92 cells to the trophoblast cells within 24 hours. The appearance of GrA in the trophoblast cells was accompanied by a simultaneous decrease in its expression by NK-92 cells only after 96 hours of co-cultivation. The data obtained indicated a predominantly early (24 hours) transport of GrB. In the process of serial killing, NK cells lose most of their perforin and GrB [16]. We did not notice a significant decrease in the level of perforin in the NK-92 cells in the co-culture with trophoblast cells after 24 hours, but we did observe a decrease after 96 hours. This indicates the active synthesis of perforin by NK cells to maintain cytotoxic capabilities on the first day of contact with trophoblasts, accompanied by a reduction in the activity of perforin synthesis after long-term co-cultivation.
The activity of granzymes is blocked by protease inhibitors or serpins. For example, serpin B9 blocks GrB[95]. It has been demonstrated that a high content of serpin B9 in CD8+ T lymphocytes correlates with the increased cytotoxicity of these cells [120]. This is attributed to the fact that cells expressing serpin B9 specifically resist GrB-mediated death whilst remaining sensitive to other inducers of apoptosis [94]. Serpin B9 has been observed to prevent NK cell death due to autotoxicity during viral infections; an increase in the GrB-mediated apoptosis of NK cells was observed in mice knocked out by serpin [94].
We discovered the presence of serpin B9 in the NK-92 cells. This concurred with the data in the literature (Table 1). We also observed that the trophoblast cells of the JEG-3 line spontaneously expressed serpin B9. According to literature data, we did not observe any expression of serpin B9 by the trophoblast cells, including the JEG-3 cells. Despite the transfer of granzymes to the trophoblast cells, there was no decrease in the viability of the trophoblast cells in our model. This supports the existence of a strategy protecting the trophoblast from the excessive cytotoxic effects of NK cells, including the activity of serpin B9. It has previously been established that trophoblasts can use the mechanism of the limited activation of caspase-8 and caspase-3 to initiate syncytium formation [121]. The assumption that trophoblast cells apply a restriction to granzyme activity using serpin B9 to initiate their own syncytialization requires further experimental confirmation. Considering the predominantly early (24 hours) transport of granules containing GrB and perforin to the trophoblasts and the later (96 hours) transport of granules containing GrA, it is plausible that there is a different degree of participation of these proteins in the activation of caspases in trophoblast cells. The expression of serpin B9 by trophoblast cells indicates the presence of a programmed resistance of these cells to the cytotoxic action of NK cells and cytotoxic T-lymphocytes. This may trigger the mechanisms of the induction of peripheral tolerance in the maternal-fetal interface. We observed that under co-culture conditions, the content of serpin B9 in the NK-92 cells decreased; it did not change in the trophoblast cells. This effect was detected after incubation for both 24 and 96 hours. None of the cytokines used canceled the inhibitory effect of trophoblasts on the expression of serpin B9 by the NK cells. Consequently, trophoblasts may increase NK cell death caused by autotoxicity after reducing the synthesis of serpin B9 [94]. This may be the basis for limiting the excessive cytotoxicity of NK cells.
A large number of cytokines are produced in the area of uteroplacental contact. Currently, there are insufficient data on their effect on trophoblast cells and NK cells under the conditions of their long-term co-cultivation. We observed that TGFβ had no effect on the expression of GrA, GrB, perforin, and serpin B9 in trophoblast and NK cells after their 24-hour incubation in mono- and co-culture conditions. After 96 hours of co-cultivation, the content of GrA in the trophoblasts increased in the presence of TGFβ. There was no decrease in the GrA content in the NK-92 cells co-cultured with JEG-3 cells but without cytokines. Thus, TGFβ supported the production of GrA by the NK cells under the conditions of long-term interactions, contributing to its enhanced transport to the trophoblasts. TGFβ is an anti-inflammatory cytokine that promotes the differentiation of regulatory NK cells, reducing their cytotoxicity[122] and changing the ability of trophoblast cells to invade [123]. The stimulation of increased GrA transport by TGFβ from NK cells to trophoblasts may be a mechanism for the activation of caspase-8 and caspase-3, which is limited under the action of serpin B9, to initiate syncytium formation by trophoblasts [124]. This hypothesis also requires experimental confirmation. The source of TGFβ for such a process in the uteroplacental contact zone may be the trophoblast cells themselves [38, 39] or the main cells of the microenvironment: macrophages [125, 126] and NK cells [36, 37].
We observed that in the monoculture of the NK cells, cytokines TNFα, IFNγ, IL-15, IL-18, IL-10, and TGFβ1 did not affect the expression of GrA, GrB, perforin, or serpin B9. We observed that IL-10 reduced the content of serpin B9 in trophoblast cells.
It has been demonstrated that proinflammatory cytokines IL-15 [127], TNFα [28], IL-18 [48], and IFNγ [28] stimulate the cytotoxic activity of NK cells. IL-10 can also indirectly stimulate the cytotoxicity of NK cells through the stimulation of IFNγ production[128]. We observed that neither TNFα nor IFNγ in co-culture conditions affected the expression of serpin B9 by trophoblast cells, indicating that they do not reduce the rate of protection of the trophoblasts in inflammatory conditions from the cytotoxic action of killer cells. TNFα canceled the inhibitory effect of trophoblasts on the expression by NK cells of proteins GrA and GrB; IL-15 had the same effect on the expression of GrA by NK cells. With long-term co-cultivation (96 hours), IL-15 increased the content of GrA in the trophoblasts; IL-10 and IFNγ increased the content of GrB in the trophoblast cells. Under the conditions of trophoblast monocultivation in the presence of IL-15 and IL-10, the serpin B9 expression decreased; under the conditions of co-culturing, the NK cells canceled the inhibitory effect of cytokines on the serpin B9 expression by the trophoblast cells. Thus, the data obtained after co-cultivation indicated the maintenance of the expression of serpin B9 by trophoblast cells with the increased transport of granzymes from NK cells in the presence of most of the pro- and anti-inflammatory cytokines used. This could help trophoblasts to maintain resistance to the cytotoxic action of killer cells under the conditions of an inflammatory process.
One of the mechanisms that trophoblast cells use to avoid the cytotoxic effect of killer cells is their expression of molecules of the nonclassical main histocompatibility complex HLA-G [80]. We observed that the cells of the JEG-3 line expressed HLA-G; this coincided with the literature data [129]. The binding of HLA-G on the trophoblast surface by the NK cell receptor KIR2DL4 [130] suppresses the cytotoxic activity of killer cells [130]. We observed that under short-term (24 hour) co-culture conditions with NK cells, the expression of HLA-G by the JEG-3 cells did not change. Of all the used cytokines, only TGFβ and TNFα affected the expression of HLA-G by trophoblast cells. TGFβ did not affect the expression of HLA-G by JEG-3 cells under monocultivation or co-cultivation for 24 hours. TGFβ increased its expression by trophoblast cells in the monoculture but not in the co-culture after 96 hours. These results confirmed the participation of TGFβ in the creation of a microenvironment that protects fetal cells. The obtained results also demonstrated the ability of natural killer cells to cancel the TGFβ effect of the stimulation of the HLA-G expression by trophoblasts under the conditions of prolonged co-cultivation. The expression of HLA-G by the trophoblast cells increased in the presence of TNFα after long-term cultivation (96 hours) in co-culture conditions. This suggests the existence of protective mechanisms of the trophoblast cells in conditions of inflammation, which are necessary to avoid an immune response of the maternal organism.
The interaction between NK-92 cells and JEG-3 cells was mainly accompanied by the early (24 hours) transport of GrB and perforin to trophoblast cells and the later (96 hours) transport of GrA to trophoblast cells. Only the expression of GrB decreased in the NK cells after 24 hours of co-cultivation; the expressions of perforin and GrA only decreased after 96 hours. The transfer of perforin and granzymes from the NK cells to the trophoblast cells was not accompanied by a decrease in their viability. This may have been due to the spontaneous expression of serpin B9 by the trophoblast cells of the JEG-3 line that we established. Trophoblasts reduced the expression of serpin B9 by the NK cells, indicating that they could increase the probability of NK cell death due to autotoxicity and trigger the restriction of the excessive cytotoxicity of natural killers as well as peripheral tolerance in the mother–fetus system. Trophoblasts retained a serpin B9 expression under the conditions of granzyme transport from the NK cells in the presence of most of the pro- and anti-inflammatory cytokines used. Under co-culture conditions, the NK cells canceled the inhibitory effect of IL-15 and IL-18 cytokines on the level of serpin B9 in the trophoblast cells. This may have contributed to the resistance of the trophoblasts to the cytotoxic action of the NK cells during the inflammatory process. Under the conditions of a long-term interaction, TGFβ supported the production of GrA by NK cells, contributing to its enhanced transport to the trophoblasts. In the presence of TGFβ, the NK cells also canceled the stimulating effect of these cytokines on the expression of HLA-G by trophoblasts, which increased the control of the NK cells over the trophoblast invasion. TNFα stimulated the expression of HLA-G by the trophoblast cells under the conditions of a long-term co-cultivation with NK cells, increasing its protective properties against excessive NK cell activity.
Conclusion. In summary, the interaction between NK and trophoblast cells is a dialogue in which the trophoblast receives cytotoxic proteins from NK cells without reducing its own viability and uses the effects of TGFβ to increase surveillance of NK cells. NK cells reduce the effect of the pro-inflammatory cytokines IL-15 and IL-18 and increase the expression of serpin B9 by trophoblasts. This leads to the inhibition of the activity of granzymes transported to trophoblasts.
Financial support
This study was funded by the project "Development of diagnostic criteria for predicting and overcoming reproductive losses", head O.N. Bespalova. State registration number 122041500061-8.





















Reference lists